Can bacteria cause cancer?
Ever since 1890, when pathologist William Russell described “a characteristic
organism of cancer,” there has been a small but dedicated group of scientists
who have claimed that bacteria (not viruses) cause cancer. Their reports
show an unusual microbe that can be seen microscopically in cancer tissue
and cultured from cancerous tumors and blood. Similar bacteria have
been reported in certain non-cancerous diseases as well. I use the term
“cancer microbes” to refer to the bacteria
described in this controversial and little-known area of cancer
research.
The idea that bacteria cause major forms of cancer was discarded a hundred
years ago by the medical establishment—and is still regarded
as scientific heresy. Bacteria derived from cancer are generally
considered as “laboratory contaminants” or “secondary invaders” or “opportunistic
infections” of weakened cancerous tissue. Nevertheless, this communication
provides evidence that “cancer microbes” can be demonstrated microscopically
in cancer tissue. The origin of these bacteria and
the various reproductive forms they express within the human body (in
vivo) is also discussed.
For details on the history of the cancer microbe, refer
to the “cancer bacteria” Wikipedia page, and my Internet article “The
return of the cancer parasite” (2011).
1) The pleomorphic nature of the cancer microbe
Cancer bacteria defy the established rules of microbiology. The cancer
microbe is “pleomorphic,” meaning the germ can exist and appear in more
than one form. This alleged “pleomorphism” immediately raises a century-old
controversy because most microbiologists do not
believe in bacterial pleomorphism. On the contrary, they believe
bacteria are monomorphic, meaning they reproduce by simply
dividing into two separate and similar appearing halves.
For more information on the monomorphism/pleomorphism debate and its
relevance to cancer microbe research, consult Milton Wainwright’s essential
Internet article “Extreme pleomorphism and the bacterial life cycle:
A forgotten controversy” (1997).
According to cancer microbe scientists, the cancer microbe may appear
in lab culture as ordinary type bacteria, such as staphylococci, streptococci,
cocco-bacilli, and rarely as TB-like mycobacteria. Needless to say,
such a proposed pleomorphic germ would be difficult, if not impossible,
for most microbiologists to accept.
Further complicating the matter is research showing that
cancer bacteria are capable of producing tiny sub-microscopic
virus-like and mycoplasma-like forms, as well as large fungal-like forms
known as “large bodies.” Such claims are anathema to the scientific
world. Nevertheless, the recognition of extreme growth forms
of the cancer microbe, as well as the complex “life cycle” attributed
to it, are essential to try and make sense out of the proposed microbiology
of cancer.
Cancer microbes can assume different forms within the body because they
are “cell wall deficient forms” (also called L-forms). The absence
of a bacterial cell wall causes a loss of rigidity and results in organisms
assuming a variety of shapes and sizes. When various species of bacteria
are in the cell wall deficient state, they cannot be distinguished from
one another.
Wainwright cautions us to pay attention to pleomorphic bacteria.
“The literature on extreme pleomorphism remains intriguing, and some
aspects of it may be worthy of reappraisal. By merely dismissing it,
we may be ignoring something of fundamental importance. This is especially
likely since examples of extreme variation in bacterial morphology continue
to be linked with various diseases and cancer in animals and humans.”
2) Cancer microbes and “acid-fast” bacteria
My mentor Virginia Livingston (1906-1990), undeniably the
leading proponent of cancer microbe research, first discovered tuberculosis-type
acid-fast staining bacteria in 1947 in scleroderma, a sometimes fatal
autoimmune connective tissue disease that causes hardening of the skin.
Her research quickly led to the finding of similar
pleomorphic bacteria in cancer and in other diseases.
I met Livingston shortly after my independent discovery of pleomorphic
acid-fast bacteria in scleroderma in 1966. A lifelong friendship ensued
and I was able to confirm some of her cancer discoveries, particularly
the identification of bacteria within scleroderma
and cancerous tissue.
Through my association with several noted microbiologists
who had extensively studied cell wall deficient bacteria,
I learned about pleomorphic forms of acid-fast mycobacteria, particularly
the round staphylococcal-like forms that were considered an additional
growth form of mycobacteria. As a result of this somewhat esoteric scientific
knowledge, I was able to confirm and report the presence of these coccoid
forms in vivo in skin biopsy material from cancer, AIDS, and certain
diseases of unknown etiology. In addition, I discovered similar
coccoid forms in the internal organs and connective tissue in autopsy
cases of scleroderma, lupus, lymphoma, AIDS, and non AIDS-related
Kaposi’s sarcoma.
3) The microscopic detection of the cancer microbe in vivo
Traditionally, bacteria in diseased tissue can
be detected with a so-called Gram stain. However, because cancer microbes
in vivo have defective or absent cell walls, they
do not stain well with the Gram stain. It is well-accepted that cell
wall deficient forms of bacteria are notoriously difficult to stain.
Also the traditional “hematoxylin and eosin” stain, routinely
used by pathologists to diagnose disease in tissue biopsy sections,
does not stain cancer bacteria.
One of Livingston’s great discoveries
was that the cancer microbe could be identified in tissue (and in culture)
by use of the “acid-fast stain,” the traditional stain used
to detect the acid-fast (red-staining) rod forms of mycobacteria
that cause tuberculosis. It is not unusual for certain microbes and
fungi to require special staining for detection. Bacteria that cause
stomach ulcers, Legionnaire’s disease, and syphilis, are a few
examples where special staining is required.
By use of the acid-fast stain, the cancer microbe appears primarily
as purple-stained variably-sized, round coccoid forms similar
to the size and shape of ordinary staphylococci. These must be viewed
at the highest magnification of the microscope and with the use of the
oil-immersion lens, which magnifies 1000 times. The bacteria are seen
within cells in grape-like and tightly packed clusters. They can also
be found singly and in small groups scattered
around the connective tissue.
Because the bacteria are cell wall deficient, it is possible to occasionally
encounter very large globular forms of the microbe. Due to their size,
these forms can be confused with fungal bodies and spores
and are consistent with what microbiologists call “large bodies.”
These forms in vivo can attain the size of red blood cells
and even larger.
One wonders how scientists can still believe exclusively in monomorphism
when modern laboratory investigations show these pleomorphic large
forms are an integral part of the reproductive “life cycle” of various
bacteria. (In this regard, see the work of the late Lida Mattman and
Gerald Domingue, both experts on cell wall deficient bacteria, and the
current work of Nadya Markova of the Institute of Microbiology,
in Sofia, Bulgaria.)
It is my belief that large bodies in vivo are similar to what Scottish
pathologist William Russell again reported in The Lancet in 1899
as “the parasite of cancer.” Unfortunately, his research was discredited
over a century ago at a time when cell wall deficient bacteria and large
bodies were unknown to microbiologists. Modern pathologists recognize
“Russell bodies” in diseased tissue, but consider them non-microbial
in nature. No attention has been paid to these forms as possible large
forms of cell wall deficient bacteria. For details on Russell, see “The
Russell body: The forgotten clue to the bacterial cause of cancer” (2003),
at the www.joimr.org website.
There are three pleomorphic forms that can be encountered in the microscopic
search of specially-stained tissue sections for cancer microbes.
These are 1) the acid-fast rod forms typical of mycobacteria; 2) the
intracellular and extracellular round coccoid forms; and 3) the “large
body” forms.
During my years of microscopic observations, I found typical tuberculosis-type
acid-fast rod-shaped bacteria in scleroderma,
and also within the tumor of an immunoblastic sarcoma (a connective
tissue tumor) in a gay man with terminal AIDS. These rod-shaped
bacteria in tissue are very rare and can require many hours of microscopic
study to demonstrate them.
However, the coccoid forms are the most prevalent
and easy-to-find forms. They can be seen tightly or loosely packed within
a cell, or in grape-like configurations, or scattered singly around
the tissue. The large bodies are infrequently encountered. Currently,
coccoid forms and large bodies are not recognized or reported by pathologists
as microbial growth forms.
The microbe is easiest to detect in scleroderma. Figures 1-3 illustrate
rare acid-fast TB-like rod-forms, frequent coccoid forms lying “naked”
in the collagen portion of the skin, and uncommon ghost-like large body
forms in the fatty portion of the skin. Figure 4. shows the pleomorphic
mycobacteria cultured from scleroderma skin in a severe and ultimately
fatal reported case. Note both the acid-fast red-stained rod forms and
the non-acid-fast blue-stained coccal forms of this organism. Figures
4-10 show the common intracellular and extracellular coccoid forms encountered
in the tissue of breast cancer, prostate cancer, Hodgkin’s lymphoma,
AIDS-related Kaposi’s sarcoma and lung cancer.
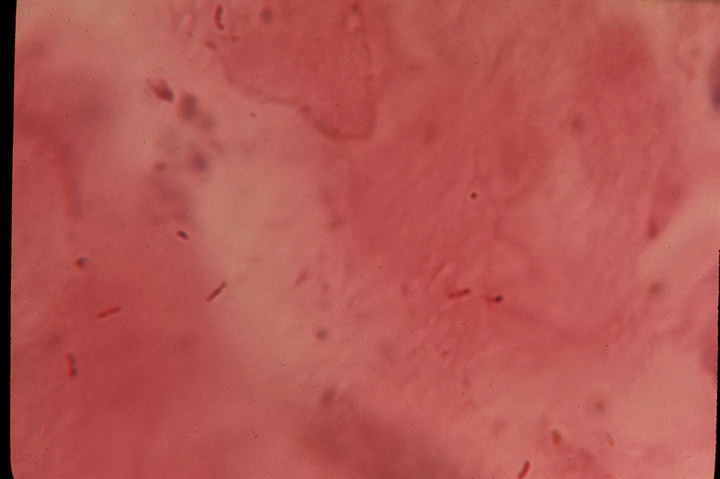
Fig 1. Rare acid-fast rod forms of mycobacteria
in the dermis portion of the
skin in scleroderma. Acid-fast stain, magnification x1000, in oil.
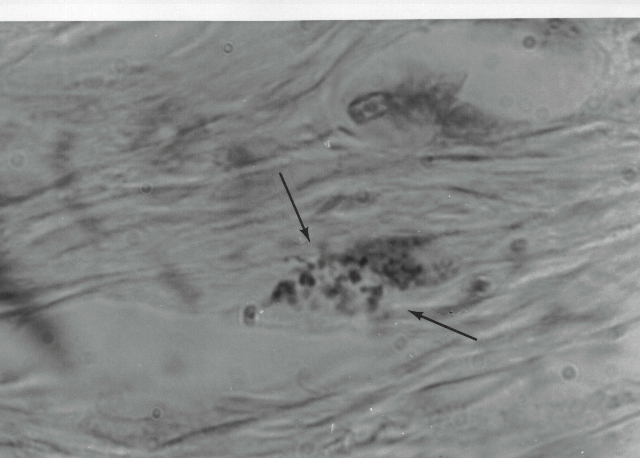
Fig 2. Coccoid forms in the dermis of the skin
in scleroderma. Acid-fast stain, x1000.
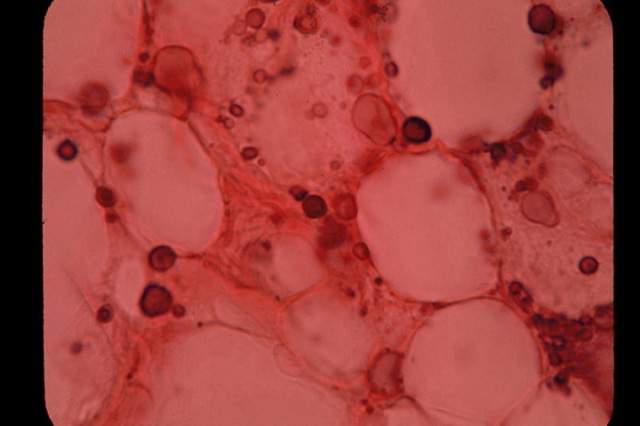
Fig 3. Variably-sized, clear, ghost-like “large
body” forms of pleomorphic bacteria
in the fatty portion of the skin in scleroderma. Acid-fast stain, x1000.
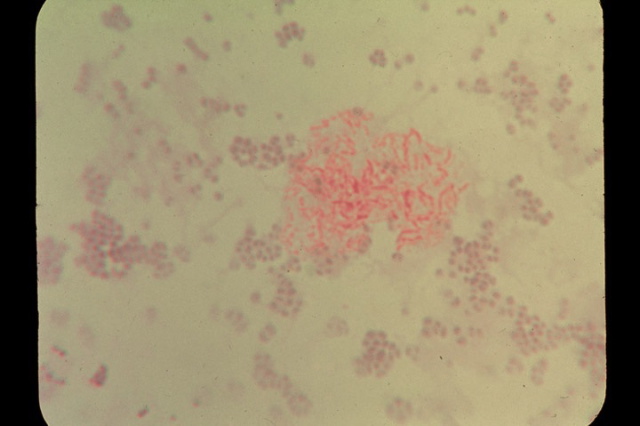
Fig 4. Smear from scleroderma skin culture of
pleomorphic acid-fast mycobacteria
showing red-stained rod forms along with non-acid-fast blue-stained
round coccal forms. Acid-fast stain, x1000.
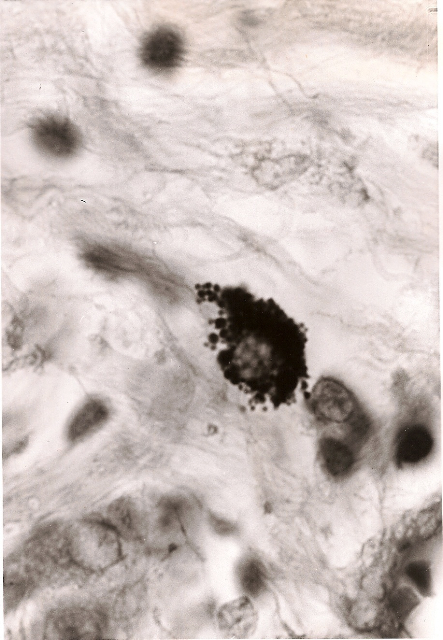
Fig 5. Breast cancer showing tightly-packed,
variably-sized
coccoid forms within a cell. Acid-fast stain, x1000.
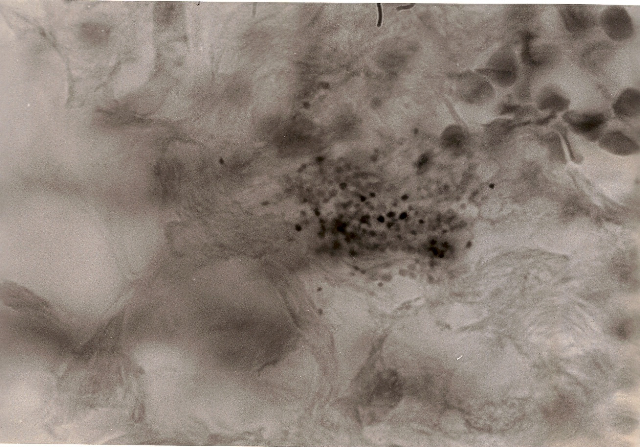
Fig 6. Breast cancer showing extracellular,
loosely-packed, variably-staining coccoid
forms in the connective tissue. In the upper right of the photo are
red blood cells. Note
the size of the tiny coccoid forms as compared to the size of the blood
cells. Acid-fast stain, x1000.
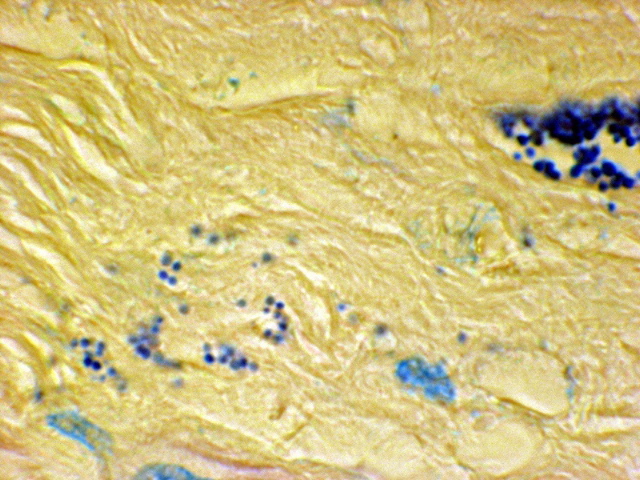
Fig 7. Prostate cancer showing a clump of grape-like
coccoid forms on the right
and scattered coccoid forms in the stroma on the left. Acid-fast stain,
x1000.
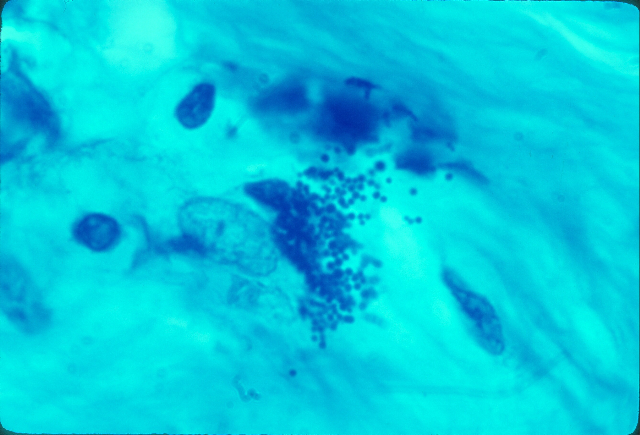
Fig 8. Hodgkin’s lymphoma. Variably-sized
coccoid forms bursting out of a
cell in the connective tissue at autopsy. Acid-fast stain, x1000.
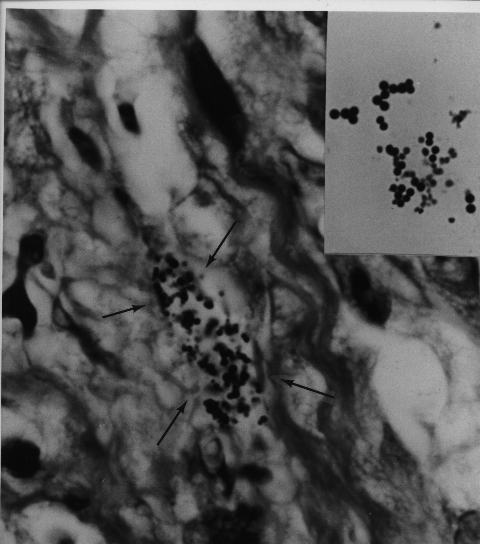
Fig 9. AIDS-related Kaposi’s sarcoma of
the skin. A collection of coccoid forms in
the dermis of the skin. Insert shows Staphylococcus epidermidis cultured
from this lesion.
Note the similar size and shape of the microbe cultured to that of the
coccoid forms
seen in vivo in the tumor. Acid-fast stain, x1000.
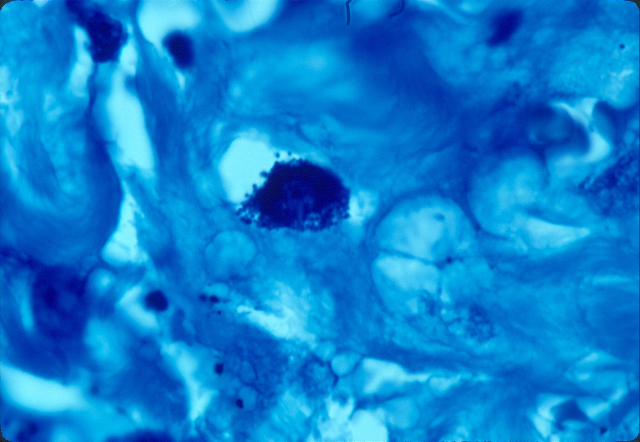
Fig 10. Lung cancer showing a cell tightly packed
with coccoid forms. Acid-fast stain, x1000.
4) The cancer microbe cannot be identified as a single “species”
For over a century bacteriologists have classified laboratory
bacteria into specific groups and species. However, current genetic
testing and molecular techniques indicate that a precise taxonomic
classification scheme for bacteria may no longer be tenable. The reason
is that bacteria, particularly within the body, are
constantly swapping genes with one another. A precise classification
of bacteria grown in the laboratory requires stable growth characteristics
and morphology which cancer microbes do not possess.
Livingston believed cancer bacteria most closely resembled the fungal-like
mycobacteria (“myco” means fungus); and in the 1970s she “classified”
them with the “actinomycetes”, a heterogeneous group of branching fungal-like
bacteria which includes the mycobacteria that cause tuberculosis.
In Wainwright’s paper on extreme pleomorphism, he notes that actinomycetes
are exempt from the monomorphic view. “Exceptions to this rule are accepted
in certain so-called higher bacteria, including some actinomycetes.”
5) The cancer microbe and the human bacterial microbiome
Cancer microbes are ubiquitous and undoubtedly related to the trillions
of bacteria normally contained within the human body. This mass of
microbes, primarily bacteria, is now called the human microbiome. Only
recently (beginning in 2008) has this microbial collective undergone
preliminary study. Now some microbiologists refer to the human body
as a “superorganism.” (For details, consult the Human Microbiome Project
in the Wikipedia).
Cancer bacteria are also related to a host of equally controversial
pleomorphic bacteria that exist in all human blood. These
blood bacteria comprise a number of different species including staphylococci,
streptococci, cocco-bacillary microbes, and others.
For more than a century, scientists have believed that human blood is
“sterile” under normal conditions. Livingston always referred to cancer
bacteria as “symbionts.” These symbionts are part of the human microbiome.
The precise affect of these trillions of body bacteria on human
disease has never been studied..
6) The arguments against the cancer microbe
When the bacterial cause of three major diseases (TB, leprosy,
and syphilis) was discovered a century ago, it was assumed that bacteria
would also be identified in cancer. But consensus opinion was
that cancer was neither contagious or infectious; and no
consistent species of bacteria could be isolated from cancer tumors.
A century later, it is widely believed that infectious agents,
particularly viruses, can cause some forms of cancer.
To this day, however, one can encounter scattered
reports of bacterial involvement in certain forms of cancer, but this
has done little to change the prevailing view that bacteria are not
a major cause of cancer. One notable exception has been the recent acceptance
of stomach bacteria (called Helicobacter pylori) as the cause of stomach
ulcers and secondary stomach cancer. There is even a new pathologic
disease called “Russell body gastritis” associated with H. pylori infection.
The current view is that Russell bodies are immunoglobulins and the
bodies are not microbial in nature, even though they are present in
conjunction with H. pylori infection. H. pylori is also a pleomorphic
microbe, exhibiting spiral, coccoid , and “degenerative forms” (Anderson
and Rasmussen, 2009).
There has also been a great deal of “mycoplasma” research in human
disease. Mycoplasma, by definition, are submicroscopic cell wall
deficient bacteria. They are the tiniest virus-like forms of bacteria,
invisible in the light microscope due to their small size. Their role
in cancer is considered speculative. I believe mycoplasma research is
closely related to cancer microbe research because cancer bacteria are
filterable and are virus-sized in certain stages of their growth. Such
forms can only be visualized by use of the electron microscope. (See,
“A history of cancer bacteria research” at www.cancerbacteria.com)
7) What causes cancer microbes to act up?
The fact that 100 trillion potentially infectious bacteria
can live symbiotically and in harmony with the ten trillion human cells
of our body is indeed miraculous. Undoubtedly the
immune system plays a major role in keeping these bacteria from becoming
microbial terrorists, but every cell in the body must also play a role,
however minor.
The first microscopic sign of disease is cellular
inflammation; and where there is inflammation there must be bacteria.
The induction of pathology is undoubtedly a multi-factorial process,
but our body bacteria are indeed opportunists involved
along with the cellular changes.
8) Cancer viruses and cancer bacteria
The viral (not bacterial) cause of cancer has been extensively studied
for the past half century. Is cancer microbe research related to cancer
virus research? We know that bacteria, like human cells, can be infected
with viruses. Is there a “connection” between the smallest virus-like
forms of cell wall deficient bacteria and “true” viruses? The precise
answers must await more recognition and study of the viral-like
and filterable growth stages of the cancer microbe.
For example, a newly discovered herpes-type virus has been declared
to cause Kaposi’s sarcoma, but reports of pleomorphic bacteria in KS
have been generally ignored. Viruses have been reported in prostate
cancer and breast cancer, but so have bacteria. TB-type pleomorphic
bacteria have been reported in AIDS, but HIV is considered the sole
virus cause of this immune disease. (See my article, “Do TB-type bacteria
cause AIDS?” )
9) How can these cancer microbes be eliminated?
Over the years I have been repeatedly asked how to eliminate (or at
least suppress) these cancer-associated bacteria. Unfortunately, I don’t
know. Livingston, who died in 1990, used a treatment regimen which included
an “autogenous vaccine” made from the patient’s own cancer bacteria.
However, she was repeatedly harassed for this by the medical establishment
and was often labeled a “quack.” In her book “The Conquest of Cancer”
(1984), she defended her therapies as ways to stimulate the immune system
against the build-up of these bacteria.
Currently, biomedical scientist Trevor Marshall has proposed a treatment
regimen for chronic disease, but not for cancer. This “Marshall
Protocol” has also been condemned by some and praised by others. Obviously,
there will always be resistance and criticism unless a disease treatment
is officially approved by the powers that be. Nevertheless, Marshall
bases his recommendations, in part, on a deep understanding of
the Human Microbiome and its potential to contribute to chronic illness.
Physicians still consider scleroderma an autoimmune connective
tissue disease of unknown cause, despite reports of acid-fast bacteria
in this disease from three different research groups. We now know
that scleroderma patients have a higher risk for cancer. Such
a connection would not have surprised Livingston who reported
acid-fast bacteria in both diseases. Infection with acid-fast mycobacteria
is also common. One-third of the world’s population is thought
to be infected with acid-fast bacteria that cause tuberculosis; and
31% of people worldwide suffer from some sort of cancer. Over the past
two decades the use of long-term, low dose antibiotic therapy
seems to help some scleroderma patients. But there are negative reports
as well.
10) The Internet and cancer microbe research
As a result of my intense interest in the microbiology of cancer over
the decades, I have written five books, thirty medical papers
in peer-reviewed journals, and numerous articles posted on the Internet.
They are all available for study. Simply Google: Cancer microbe research.
In addition, one can Google the findings of cancer
microbe researchers of the past who have inspired me, particularly
William Russell, Wilhelm Reich, Raymond Royal Rife, Virginia Livingston,
Eleanor Alexander-Jackson, Irene Corey Diller, Florence Seibert, and
others.
Their discoveries should be studied by physicians,
particularly oncologists, pathologists and microbiologists. Continuing
to ignore an entire body of cancer microbe research is a disservice
to patients, and not in the best interest of good science.
--------
Alan Cantwell is a retired dermatologist. He is the author of “The Cancer
Microbe” and “Four Women Against Cancer,” available on Amazon.com.
His scientific papers can be found on the PubMed website (Use “Cantwell
AR” in the search engine). For his Internet papers, Google: “alan cantwell”
+ articles. E-mail: alancantwell@sbcglobal.net. Website: www.AriesRisingPress.com
|
![]()









