- I have long wondered why prostate cancer was NOT a common
cancer in male AIDS patients who have been on the "AIDS cocktail"
(and sometimes added male hormones) for almost two decades, particularly
when HIV is considered to be a "cancer-causing virus."
-
- As the report below suggests, maybe HIV drugs are "protective"
and the reason that PROSTATE CANCER is NOT a common cancer in ageing HIV/AIDS
patients!!! (UNLIKE OTHER FORMS OF CANCER -- like Kaposi's sarcoma, various
lymphomas, cervical cancer, etc -- which ARE
- cancers commonly associated with AIDS. )
-
- Also some AIDS patients are given testosterone "replacement"
hormone therapy to treat the "wasting syndrome" sometimes associated
with chronic AIDS. Doctors usually hesitate to give healthy ageing males
"extra" testosterone for fear of CAUSING prostate cancer!! (There
is recent evidence that the PROHIBITION of treating prostate cancer WITH
testosterone is now being CHALLENGED -- see Morgentaler's partial paper
from March, 2010, at the end of this email)
-
- As you may know, "acid-fast" cell wall deficient
pleomorphic BACTERIA have been discovered and reported in BOTH AIDS and
prostate cancer. Although this research of mine has been ignored by the
AIDS establishment (with HIV the supposed SOLE cause of AIDS) -- it may
eventually prove that "HIV drugs" (and hormone therapy??) also
have some killing or suppressive effect on the bacteria reported in prostate
cancer and AIDS.
-
- ALAN CANTWELL M.D.
-
- for more info on bacteria in AIDS and prostate cancer.....
-
- GOOGLE: Cantwell + prostate cancer
-
- and/or Cantwell + AIDS bacteria
-
-
- HIV Drugs Might Combat Two Other Diseases
- Prostate cancer, chronic fatigue are new research targets
-
- (HealthDay News) -- Four anti-HIV drugs inhibit a retrovirus
recently linked to prostate cancer and chronic fatigue syndrome (CFS),
say U.S. researchers.
- If further investigation proves that the retrovirus xenotropic
murine leukemia virus-related virus (XMRV) causes prostate cancer or CFS,
these HIV drugs may be an effective treatment for the two conditions.
-
- In this study, researchers from the University of Utah
and Emory University/Veterans Affair Medical Center tested how effectively
45 compounds used to treat HIV and other viral infections worked against
XMRV. Raltegravir was the most effective, and three other drugs -- L-00870812,
zidovudine (ZDV or AZT), and tenofovir disoproxil fumarate (TDF) -- also
prevented XMRV replication.
-
- "Our study showed that these drugs inhibited XMRV
at lower concentrations when two of them were used together, suggesting
that possible highly potent 'cocktail' therapies might inhibit the virus
from replicating and spreading," Raymond F. Schinazi, a professor
of pediatrics and chemistry and an investigator with the Center for AIDS
Research at the Emory University School of Medicine and the Atlanta VA,
said in a news release.
-
- "This combination of therapies might also have the
added benefit of delaying or even preventing the virus from mutating into
forms that are drug-resistant," Schinazi added.
-
 "These results offer hope to infected persons, but
we are still at the early stages of our understanding of the potential
link between XMRV and these diseases," Dr. Ila R. Singh, an associate
professor of pathology at the University of Utah Medical School, said in
the news release. "These results offer hope to infected persons, but
we are still at the early stages of our understanding of the potential
link between XMRV and these diseases," Dr. Ila R. Singh, an associate
professor of pathology at the University of Utah Medical School, said in
the news release.
-
- The study was published April 1 in the journal PLoS One.
-
- More information
-
- The U.S. Centers for Disease Control and Prevention outlines
the possible causes of chronic fatigue syndrome.
-
- -- Robert Preidt
-
- SOURCE: University of Utah Health Sciences, news release,
April 1, 2010
-
- Copyright © 2010 HealthDay. All rights reserved.
-
-
- Alan Cantwell M.D.
- alancantwell@sbcglobal.net
-
- author of THE CANCER MICROBE
-
- www.ariesrisingpress.com
-
-
-
- Oncol Rep. 2007 May;17(5):1121-6.
- HIV infection and cancer in the era of highly active
antiretroviral therapy (Review).
- Barbaro G, Barbarini G.
- Department of Medical Pathophysiology, University La
Sapienza, 00174 Rome, Italy. g.barbaro@tin.it
-
- Abstract
- The majority of cancers affecting HIV-infected subjects
are those established as acquired immunodeficiency syndrome (AIDS)-defining:
Kaposi's sarcoma (KS), non-Hodgkin's lymphoma (NHL), and invasive cervical
cancer (ICC). However, other types of cancer, such as Hodgkin's disease
(HD), anal cancer, lung cancer and testicular germ cell tumors appear to
be more common among HIV-infected subjects compared to the general population.
While not classified as AIDS-defining, these malignancies have been referred
to as AIDS-associated malignancies. The mechanisms by which depressed immunity
could increase the risk for cancer are unclear, except for in KS and most
subtypes of NHL, where it is strictly associated with a low CD4 count.
Although it remains unclear whether HIV-1 acts directly as an oncogenic
agent, it may contribute to the development of malignancies through several
mechanisms (e.g., infection by oncogenic viruses, impaired immune surveillance,
imbalance between cellular proliferation and differentiation). Studies
of the effect of highly active antiretroviral therapy (HAART) on the incidence
and progression of HIV/AIDS-associated cancers provided contrasting data.
While a significant decrease in the incidence of KS has been observed,
HAART has not had a significant impact on NHL incidence, particularly systemic
NHL, or on ICC, HD, anal cancers and other non-AIDS-defining cancers. Regardless
of whether these cancers are directly related to HIV-induced immunodeficiency,
treating cancer in HIV-infected patients remains a challenge because of
drug interactions, compounded side effects, and the potential effect of
chemotherapy on CD4 count and HIV-1 viral load. A better knowledge of viral
mechanisms of immune evasion and manipulation will provide the basis for
a better management and treatment of the malignancies associated with chronic
viral infections.
- PMID: 17390054 [PubMed - indexed for MEDLINE]
-
- J Natl Med Assoc. 2008 Jul;100(7):817-20.
- Malignancies in HIV: pre- and post-highly active antiretroviral
therapy.
- Nutankalva L, Wutoh AK, McNeil J, Frederick WR, Reddy
RB, Daftary M, Gentles A, Addae-Afoakwa K.
- College of Medicine, Howard University, Washington, DC
20059, USA.
-
- Abstract
- OBJECTIVES: A study was conducted at a large metropolitan
tertiary-care teaching hospital to investigate the incidence of cancers
among HIV-infected patients over a 13-year period. DESIGN: Retrospective
cohort study. METHODS: A retrospective cohort study was conducted among
HIV-infected patients diagnosed with cancer between January 1990 and December
2003 at a large metropolitan teaching hospital. Any HIV-infected patient
who also had a confirmed diagnosis of Kaposi's sarcoma, primary central
nervous system lymphoma, invasive cervical cancer or non-Hodgkin's lymphoma
was categorized as having AIDS-defining cancer (ADC) according to the CDC's
initial case definition for AIDS, while patients with other malignancies
were classified as having non-ADCs. A clinical database was created consisting
of HIV patients diagnosed with cancer at this teaching hospital, and data
were abstracted for the current project. RESULTS: A total of 203 HIV-infected
patients diagnosed with cancer were identified during the study period.
Ninety-three cases occurred before 1995 and 110 after 1996. The median
age of patients (at cancer diagnosis) in the era before highly active antiretroviral
therapy (HAART) was 37 years and in the post-HAART era was 43 years (p<0.05).
Mean CD4 count at cancer diagnosis in the pre-HAART era was 101 cells/mm3,
and 183 cells/mm3 in the post-HAART period (p<0.05). Six patients had
diagnoses of both ADC and NADC during the study period. Of the 197 remaining
cases, 129 (65.4%) were ADCs and 68 (34.6%) were NADCs (p<0.05). The
incidence of Kaposi's sarcoma decreased significantly, while the incidence
of lung cancer increased significantly. CONCLUSIONS: Of 197 patients with
a single diagnosis of either ADC or NADC, there was statistically a larger
proportion of NADC cases diagnosed in the post-HAART period compared to
the pre-HAART period. The number of ADC diagnoses decreased between the
pre- and post-HAART period.
- PMID: 18672558 [PubMed - indexed for MEDLINE]
-
-
- March 1, 2010
- Use of testosterone therapy in hypogonadal men with prostate
cancer
- By Abraham Morgentaler, MD
-
- The long-standing prohibition against testosterone therapy
(TTh) in men with prostate cancer is now being challenged. New evidence
indicates that TTh in these men is not as risky as once believed, and a
number of studies have reported good symptomatic results from TTh without
cancer recurrence in men who were treated for prostate cancer.
-
-

- Abraham Morgentaler, MD
-
- Just a few years ago, it would have been unthinkable
to offer testosterone therapy (TTh) to men with a history of prostate cancer.
Yet several changes have occurred to make TTh (also known as testosterone
replacement therapy) a reasonable treatment option in men who are symptomatic
from testosterone (T) deficiency. The greatest stimulus has been the desire
of men to improve their health and quality of life. A re-evaluation of
the historical prohibition against TTh in men with prostate cancer has
been prompted by increased awareness of the benefits of TTh, including
improvement in sexual desire and function, energy, vitality, mood, and
physical performance.1Evidence now indicates that TTh in men with prostate
cancer is not nearly as risky as once believed2 (figure 1).
-
-
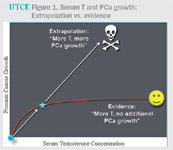
-
- Figure 1. Serum T and PCa growth: Extrapolation vs. evidence
- Indeed, a number of publications have now reported good
symptomatic results from TTh without cancer recurrence in men following
various types of prostate cancer treatments.3-7 Although the total number
of men in these studies is limited, the historical fear that higher T must
necessarily lead to prostate cancer recurrence or progression is clearly
incorrect.
-
- The relationship between T and prostate cancer has been
a primary interest of mine, and it has been fascinating to watch the transformation
in thought and practice over the last 20 years. In this article, I will
present our current understanding of this topic and its impact on clinical
practice.
-
- 'Feeding a hungry tumor'
-
- At the time of my urology residency in 1984-'88, it was
axiomatic that higher T was responsible for prostate cancer development.
My fellow residents and I learned that giving T to men with prostate cancer
was like "pouring gasoline on a fire" or "feeding a hungry
tumor." We saw with our own eyes that men who presented with bony
pain from prostate cancer metastases responded nicely to bilateral orchiectomy,
often within hours. It was obvious that prostate cancer was androgen-dependent,
and there was no reason to doubt that less T was a good thing for men with
prostate cancer. Subsequent experience, in which discontinuation of luteinizing
hormone-releasing hormone agonist treatment was associated with a steady
rise in PSA, appeared to close the circle; lowering T caused prostate cancer
to regress, and raising T caused prostate cancer to grow.
-
- After my residency, I became interested in TTh for men
with sexual dysfunction. I was impressed by how many of my patients responded
well to TTh with improved erections and libido, and was surprised when
several of my first TTh patients reported a renewed sense of vitality and
resolution of chronic fatigue. Some of these patients asked to stay on
TTh even when their ED persisted because they felt better overall. However,
I was concerned about the risk of stimulating occult prostate cancer, and
a few years later began to require prostate biopsy prior to initiating
TTh in order to exclude the presence of prostate cancer.
-
- At the time, it was believed that high T led to prostate
cancer and low T was protective against prostate cancer. So it was confusing
to all when my colleagues and I in 1996 found prostate cancer in 11 of
77 (14%) T-deficient men with normal PSA (<4.0 ng/mL), a cancer rate
in an otherwise normal population that was far higher than anything published
at the time.8 A subsequent series in 345 T-deficient men (mean age, 58
years) with PSA <4.0 ng/mL found a similar prostate cancer rate of 15%.
Moreover, men with the most severe degree of T deficiency had twice the
degree of risk as men with milder T deficiency.9 These results indicated
that low T was not protective against prostate cancer, and even suggested
paradoxically that low T may represent a risk for prostate cancer.
-
- In 2004, my colleague Ernani Rhoden, MD, and I published
a review on the risks of TTh in the New England Journal of Medicine. 10
We were stunned to discover that we could not find a single article in
the PSA era that clearly showed a link between higher serum T and prostate
cancer. On the contrary, multiple studies showed that men with higher endogenous
serum T were at no greater risk for prostate cancer than men with low endogenous
serum T. And prostate cancer rates in TTh trials were no different than
for the general population.10 Even men with prostatic intraepithelial neoplasia,
believed to be a pre-malignant condition, did not develop prostate cancer
at a worrisome rate after 12 months of TTh.11
-
- Furthermore, PSA has been shown in several population
studies to be unrelated to serum T,12 and the administration of supraphysiologic
T doses for up to 40 weeks did not cause an increase in either PSA or prostate
volume.13
-
- This was confusing. How was it possible that androgen
deprivation and its discontinuation cause such major changes in PSA, yet
so many other studies showed no influence of T on PSA, prostate volume,
or prostate cancer risk? In the archives of the Countway Medical Library
at Harvard Medical School, I found the original landmark paper from 1941
by Huggins and Hodges, which showed that castration or estrogen treatment
(which lowers T) resulted in a rapid and substantial decline in the serum
marker acid phosphatase in men with metastatic prostate cancer.14 This
was the paper that established androgen deprivation as the mainstay of
treatment for advanced prostate cancer.
-
- In addition, the authors reported that every man who
received T administration developed an elevation of acid phosphatase, causing
the authors to conclude that T caused "activation" of prostate
cancer and more rapid growth.14However, the number of men who received
T was only three, and results were only given for two men. One of these
men had already been castrated.14 The acid phosphatase curve for the hormonally
intact man was erratic. Amazingly, the origin of the general assertion
that higher T led to greater prostate cancer growth was based on equivocal
results in a single non-castrated man!2
-
- Other investigators in the pre-PSA era also reported
results of T administration in men with metastatic prostate cancer.15,16
Two quite different sets of results were seen. Men with recurrent disease
after castration uniformly did poorly with T administration, whereas T
administration in men who were previously untreated or newly castrated
appeared to have a benign course when T was administered. The authors of
one of these studies speculated that perhaps normal T concentrations were
sufficient to produce maximal prostate cancer growth.16 Unfortunately,
this prescient concept did not survive into the modern prostate cancer
era.2
-
- The saturation model: Water for a thirsty tumor
-
- It is now clear that prostate tissue, whether benign
or malignant, does not have an endless capacity for increased growth as
androgen concentrations increase. There is no doubt that prostate tissue
requires androgen for optimal growth, but once the requirement for androgens
has been satisfied, additional androgen has little, if any, further effect
on growth or PSA response.17 Prostate cancer cell lines demonstrate a dose-response
growth curve with increasing concentrations of T or dihydrotestosterone
(DHT), but then plateau and demonstrate no further growth even with log
increases in androgen concentration.17 A saturation model has been described
to account for the biphasic response of prostate tissue to androgens, with
exquisite sensitivity to androgens at very low T concentrations and indifference
to changes in androgens at higher concentrations.17-18
-
- There are at least two possible mechanisms to account
for the saturation model. One is that the androgen receptor (AR) in benign
human prostate tissue becomes maximally bound to androgen at approximately
120 ng/dL.19 Since it is the complex of androgen-AR that binds to androgen
response elements of nuclear DNA, T concentrations above this saturation
point no longer have AR-mediated mechanisms to influence growth. It is
rare for men to have naturally occurring T concentrations less than 120
ng/dL, although the saturation model would suggest that men who do might
still demonstrate prostate growth or PSA increase with additional T.
-
- Another possibility is suggested by a study in which
intraprostatic concentrations of T and DHT were measured before and after
6 months of TTh.20 Although serum T levels increased markedly, intraprostatic
concentrations of T and DHT were no different from those seen at baseline.
This suggests that the intraprostatic hormonal microenvironment is relatively
protected from changes in serum T.
-

-
- Figure 2. The saturation model of serum T and PCa growth
- I no longer describe the relationship of T to prostate
cancer as "food for a hungry tumor." Instead, I now describe
the relationship as "water for a thirsty tumor" (figure 2). Once
the thirst has been satisfied, additional water is simply excess.
-
- Experience with TTh after PCa Tx
-
- A small number of studies have investigated the effects
of TTh in men after various forms of treatment for localized prostate cancer.
Three have reported results of TTh after radical prostatectomy, with a
total of 74 men, all with undetectable PSA prior to TTh. None developed
biochemical recurrence.3-5
-
- Sarosdy reported the results of 4.5 years of TTh in 31
men following brachytherapy. None developed biochemical recurrence.6 In
addition, a small study reported no recurrences in five men who received
TTh after external beam radiation.7
-
-

-
- Figure 3. T therapy and PCa incidence: Traditional, current
views
- One possible reason that none of these men developed
cancer recurrence is because of complete eradication of cancer. Another
possibility, as suggested by the saturation model,17 is that any existing
but occult prostate cancer cells had already been maximally stimulated
by the relatively low but still quite appreciable T in the bodies of these
men (figure 3).
-
- An interesting case that lends support to this concept
is the response to TTh in an 84-year-old man with untreated prostate cancer.21
This active, elderly man underwent biopsy for a PSA of 8.1 ng/mL (repeat
8.5) revealing Gleason 6 cancer in two of six cores. He elected surveillance
for his prostate cancer and requested TTh for his sexual dysfunction. His
PSA gradually declined, and he continues on TTh 3 years later without evidence
of prostate cancer progression.
-
- Although it may seem risky to raise T concentrations
in men with untreated prostate cancer, it must be recognized that we already
treat a great many men with untreated prostate cancer, since one in seven
hypogonadal men with normal PSA has biopsy-detectable prostate cancer.8,9
Yet there is no evidence to suggest these men are at any greater risk of
developing prostate cancer than if they remained untreated. In fact, higher
T may even turn out one day to be beneficial, since laboratory studies
have shown that T promotes a less aggressive, better-differentiated phenotype
in some prostate cancer cell lines.17
-
- In fact, it is possible that we have been chasing the
wrong suspect when it comes to hormonal risk factors for prostate cancer.
Although multiple studies have failed to provide any solid data linking
high T to worrisome prostate cancer features or outcomes, a number of studies
(but not all) have reported associations between low T and aggressive features
of prostate cancer, including high Gleason grade, advanced stage at presentation,
biochemical recurrence after radical prostatectomy, and survival.22 Perhaps
we should be more concerned with low T than high T when it comes to prostate
cancer. After all, men tend to get prostate cancer when they are old and
their T concentrations are low; they never get prostate cancer during their
late teens and twenties, representing their peak T years.
-
- Summary: Putting it all together
-
- There are many beneficial effects of TTh in T-deficient
men, including improvements in sexual function, libido, vitality, and mood.
This is no less true in the T-deficient man with a history of prostate
cancer. As more and more men are diagnosed with prostate cancer, the dilemma
becomes more acute. Is it reasonable to withhold a treatment known to have
beneficial effects when the cancer risk is theoretical, unproven, and based
on observations in previously castrated men? A moderately large placebo-controlled
study sponsored by the National Institute on Aging has been initiated to
examine several aspects of TTh, but prostate cancer outcomes are not a
primary endpoint due to the relatively short time of treatment (1 year).
In the meantime, clinicians must make their own best judgment as to the
safety of TTh based on available information.
-
- In my opinion, it is reasonable to offer TTh to men with
prostate cancer who are symptomatic from T deficiency and have a PSA value
indicative of cure or of favorable prognosis after definitive cancer treatment.
However, it is critical to inform men of the limited safety data available
at the present time, and that there can thus be no guarantee regarding
safety. It must also be recognized that some men with prostate cancer will
experience disease recurrence or progression even without TTh; if a negative
outcome occurs during T treatment, one therefore cannot assume the cause
was TTh. Special caution should be exercised in men who are severely T
deficient (<200 ng/dL), since these men still may have ample remaining
potential for androgenic stimulation of existing prostate cancer cells,
especially those who have undergone androgen deprivation.
-
- Dr. Morgentaler is Director of Men's Health Boston, Brookline,
MA, and Associate Clinical Professor of Urology, Harvard Medical School,
Boston. Dr. Morgentaler discloses that he has served as a paid consultant
for Slate Pharmaceuticals; has provided promotional services for Solvay,
Watson, and Auxilium; and has conducted contracted research for GlaxoSmithKline.
-
- References
-
- 1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone
therapy in adult men with androgen deficiency syndromes: an Endocrine Society
clinical practice guideline. J Clin Endocrinol Metab 2006; 91:1995-2010.
-
- 2. Morgentaler A. Testosterone and Prostate Cancer: An
Historical Perspective On A Modern Myth. Eur Urol 2006; 50:935-9.
-
- 3. Kaufman JM, Graydon RJ. Androgen replacement after
curative radical prostatectomy for prostate cancer in hypogonadal men.
J Urol 2004; 172:920-2.
-
- 4. Agarwal PK, Oefelein MG. Testosterone replacement
therapy after primary treatment for prostate cancer. J Urol2005; 173:533-6.
-
- 5. Khera M, Grober ED, Najari B, et al. Testosterone
replacement therapy following radical prostatectomy. J Sex Med2009; 6:1165-70.
-
- 6. Sarosdy MF. Testosterone replacement for hypogonadism
after treatment of early prostate cancer with brachytherapy. Cancer 2007;
109:536-41.
-
- 7. Morales A, Black AM, Emerson LE. Testosterone administration
to men with testosterone deficiency syndrome after external beam radiotherapy
for localized prostate cancer: preliminary observations. BJU Int 2009;
103:62-4.
-
- 8. Morgentaler A, Bruning CO, III, DeWolf WC. Incidence
of occult prostate cancer among men with low total or free serum testosterone.
JAMA 1996; 276:1904-6.
-
- 9. Morgentaler A, Rhoden EL. Prevalence of prostate cancer
among hypogonadal men with prostate-specific antigen of 4.0 ng/mL or less.
Urology 2006; 68:1263-7.
-
- 10. Rhoden EL, Morgentaler A. Risks of testosterone-replacement
therapy and recommendations for monitoring. N Engl J Med 2004; 350:482-92.
-
- 11. Rhoden EL, Morgentaler A. Testosterone replacement
therapy in hypogonadal men at high risk for prostate cancer: results of
1 year of treatment in men with prostatic intraepithelial neoplasia. J
Urol 2003; 170:2348-51.
-
- 12. Monath JR, McCullough DL, Hart LJ, et al. Physiologic
variations of serum testosterone within the normal range do not affect
serum prostate-specific antigen. Urology 1995; 46:58-61.
-
- 13. Cooper CS, Perry PJ, Sparks AE, et al. Effect of
exogenous testosterone on prostate volume, serum and semen prostate specific
antigen levels in healthy young men. J Urol. 1998; 159:441-3.
-
- 14. Huggins C, Hodges CV. Studies on prostatic cancer.
I. The effect of castration, of estrogen and of androgen injection on serum
phosphatases in metastatic carcinoma of the prostate. Cancer Res 1941;
1:293-7.
-
- 15. Prout GR, Brewer WR. Response of men with advanced
prostatic carcinoma to exogenous administration of testosterone. Cancer
1967; 20:1871-8.
-
- 16. Fowler JE, Whitmore WF, Jr. The response of metastatic
adenocarcinoma of the prostate to exogenous testosterone. J Urol 1981;
126:372-5.
-
- 17. Morgentaler A, Traish AM. Shifting the paradigm of
testosterone and prostate cancer: the Saturation Model and the limits of
androgen stimulation of prostate cancer. Eur Urol 2009; 55:310-21.
-
- 18. Morgentaler A. Testosterone replacement therapy and
prostate cancer. Urol Clin N Am 2007; 34:555-63.
-
- 19. Traish AM, Williams DF, Hoffman ND, et al. Validation
of the exchange assay for the measurement of androgen receptors in human
and dog prostates. Prog Clin Biol Res 1988; 262:145-60.
-
- 20. Marks LS, Andriole GL, Fitzpatrick JM, et al. The
interpretation of serum prostate specific antigen in men receiving 5alpha-reductase
inhibitors: a review and clinical recommendations. J Urol 2006; 176:868-74.
-
- 21. Morgentaler A. Two years of testosterone therapy
associated with decline in serum prostate-specific antigen in a man with
untreated prostate cancer. J Sex Med 2009; 6:574-7.
-
- 22. Morgentaler A. Testosterone deficiency and prostate
cancer: emerging recognition of an important and troubling relationship.
Eur Urol 2007; 52:623-5.
|