An excipient is usually an inert substance, a means to an end, often improper, that forms a vehicle for a drug. As an example, dyes have been found to be carcinogenic, therefore against the law per the Delaney clause, and an old article said the FDA had postponed the issue 29 times. Dr. Jacqueline Verrett, toxicologist, at the time of the investigation of aspartame , wrote a book "Eating Maybe Dangerous to your Health" and is a great deal on the issue of dyes and the FDA. She basically says until the FDA is pushed against the wall they are not going to do anything.
Like in the issue of aspartame the FDA knows its a drug and not an additive, have Dr. H. J. Roberts medical text, "Aspartame Disease: An Ignored Epidemic" with a chapter on drug interaction, yet continues to allow new drugs with added aspartame which can interact. So many drugs contain aspartame, defeat their purpose and can kill. I stopped breathing three times after being given drugs with aspartame and didn't know it.
Number one on the FDA list of 92 symptoms is headache: http://www.mpwhi.com/92_ aspartame_symptoms.pdf As an example they put aspartame in Maxalt which is a drug for headache. The company was notified that aspartame triggers headache and they add it to a drug that treats headache. They refused to do anything about. A small study was conducted which showed taking Maxalt with aspartame makes the problem worse. They still refused to remove it. Here is a list of just a few drugs which contain aspartame: https://rense.com/general96/ wow-the-drugs-that-contain- Aspartame-rx-and-otc.php Here is the chapter on aspartame and drug interaction on this Hospital Report: http://www.mpwhi.com/ aspartame_hospital_form.htm
There is no one to protect you as the FDA themselves have explained. In 2009 some of the employees wrote to then Present Obama and discussed the FDA is broken, there is corruption and whistleblowers fear reprisals. So you're on your own, take it seriously and read labels. The life you save maybe your own. Also write your Congressman and Senator in Washington. Like Dr. Verrett said until the FDA is backed against the wall will they change. The Speaker of the House, Newt Gingrich, about a quarter of a century ago tried to assist me to get the FDA to answer 26 questions. They remain unanswered to this day: : http://www.mpwhi.com/26_ aspartame_questions.htm They simply refused. Isn't it the guilty who take the fifth!?
Read on for more information.
Dr. Betty Martini, D.Hum, Founder
Mission Possible World Health Inll
www.mpwhi.com
More information on aspartame on www.wnho.net , www.holisticmed.com/aspartame , and www.dorway.com files now on www.mpwhi.com because DORway was hacked.
https://www.futura-sciences. com/sante/actualites/ medicaments-medicaments- contiennent-excipients-pas-si- inoffensifs-ca-75375/
" The majority of medications contain inactive ingredients that can cause adverse reactions and affect the safety and security of these medications ." The findings of the MIT and Harvard Medical Schoo I researchers in Boston are unmistakable in their March 13 issue of Science Translational Medicine .
Up to 35 excipients in one tablet
The researchers analyzed 42,052 drugs from the American Library of Medicine (NLM) and discovered that 92.8% of drugs contain at least one inactive substance that can cause an allergic reaction . In total, the excipients represent 71% of the weight of a tablet, against just 29% for the active substance itself. On average, a drug contains more than eight different excipients, but the authors have counted up to 35 different formulations.
Do allergic people have to give up treatment?
For example, 45% of medicines contain lactose , a sugar that can cause allergies in sensitive people; 55% contain an oligosaccharide-type sugar, monosaccharide or polyol, which can cause intestinal disorders. Some dyes, such as tartrazine, are thought to aggravate asthma attacks .
"If these substances are, in general, too small to cause an effect, very sensitive patients can be affected, " says Daniel Reker, a co-author, at the Inverse site . " The problem for these patients is that they have to make a choice between their allergy and the illness they are being treated for ." And there is not always an alternative: 100% of progesterone treatments contain peanut, notes the researcher.
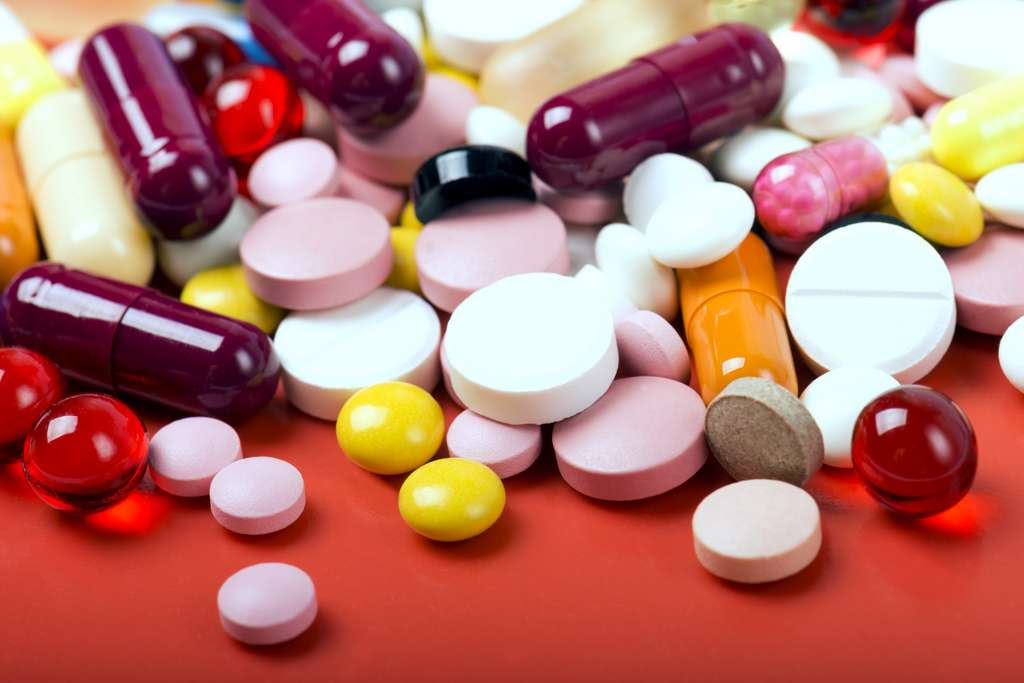
93% of drugs contain allergenic excipients, but in a crippled quantity. © ulkan, Fotolia
A study "false alarmist"
For François Chast, Honorary President of the National Academy of Pharmacy, this kind of alarmist studies simply does not make sense. " It takes 10 to 15 grams of lactose to trigger a lactose allergy. However, a drug will contain a maximum of 300 mg, "says the specialist. Tartrazine? " It is much more dangerous for an asthmatic to walk in the forest where there are plenty of pollen than to absorb a pill containing a few milligrams of tartrazine, " laughs the practitioner. The latter, however, agrees on one point with the authors of the study: the need to clean up the drug formulations. " It is quite possible to reduce preservatives or dyes in many specialties, "says François Chast.
Excipients: useful or not?
Most of the time, these substances are used for marketing purposes to give a beautiful color or a glossy coating to the tablet. But this is not always easy: in 2017, the Merck laboratory thought it best to replace Levothyrox's lactose with mannitol, an excipient with no noticeable effect. The result: an outcry of patients complaining of side effects with the new formula. In Europe, regulations require manufacturers to indicate excipients with known effect on the packaging and in the package leaflet. They number 47 in France, whose aspartame , the glucose , the starch from wheat or sesame oil.
These excipients are mentioned for information purposes because no allergic test is carried out by the National Agency for the Safety of Medicines (ANSM) which publishes this list. One or two cases of adverse effect are sufficient to mention an excipient. Also included in the package leaflet are all other excipients with no known effect, such as magnesium stearate or gelatin.
WHAT YOU MUST REMEMBER
- Of 10 drugs, 9 contain excipients that can cause allergies or intestinal disorders.
- These non-active substances represent 71% of the weight of a drug.
- The quantities contained in a tablet are, however, very insufficient to constitute a real problem.
|
