The Incredible &
Unrecognized |
By
Alan
R Cantwell, MD |
| Introduction
The cause of cancer is a germ, a microbe, a microorganism, a harmless and a disease-producing infectious agent, a blood parasite and a life form that lives within us all. It is virus-like, bacteria-like, staphylococcus-like, streptococcus-like, spore-like, and fungus-like. It is all these things and more. In suitably-stained tissue sections of cancer its bacterial and fungal-like forms are visible in a common brightfield light microscope at its highest magnification. Its smallest virus-like, mycoplasma-like, cell wall deficient forms (L-forms) can pass through a 0.22 micron lab filter designed to hold back bacteria. Its submicroscopic virus-like forms can be viewed only with a high power electron microscope.. An autofluorescent microscope, a dark field microscope and a phase contrast microscope reveal still other “pleomorphic “ forms, a word used to describe the endless sizes and shapes of this germ. This complex microbe is not found in microbiology books, nor in histopathology texts (histopathology is the microscopic study of diseased tissue). Scientists generally believe a purported “cancer microbe” does not exist; and that bacteria do not play a causative role in major forms of cancer. The “life cycle” described for the cancer germ is regarded as nonsense. But, in fact, the cancer microbe has been known for more than a century. It is best described in the category of bacteria that have lost part or all of their cell wall, the so-called cell wall deficient (CWD) bacteria. It is most closely related to CWD acid-fast mycobacteria, the kind of bacteria that causes tuberculosis (TB), leprosy, and other diseases. This short communication illustrates how the cancer germ appears in histopathologic tissue sections in vivo (within the body) and outside the body in laboratory culture (in vitro) , and how it continues to be interpreted as various “granules” and “globules” and “bodies” that mask the microbe’s true identity.
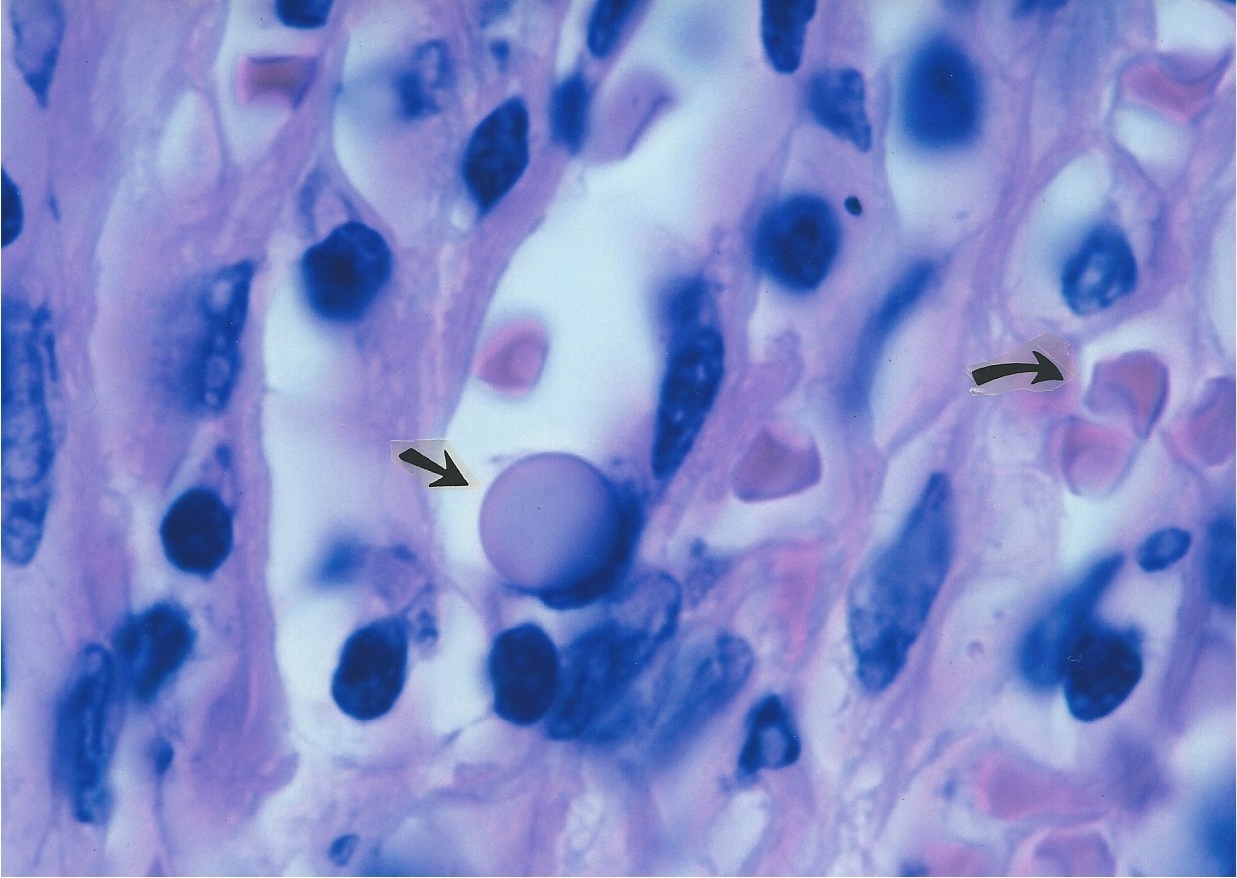
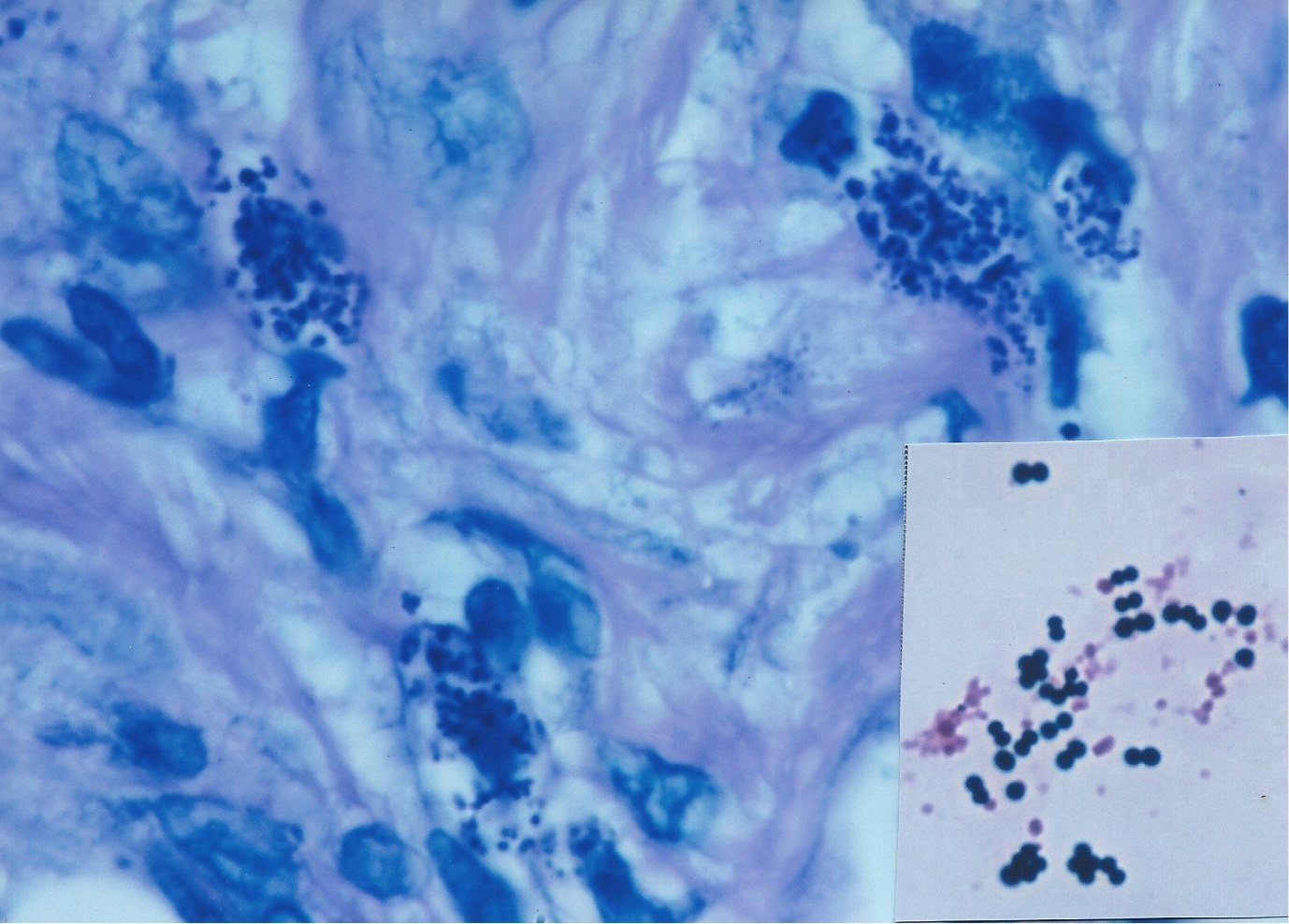
William Russell and the “characteristic microorganism of cancer” On December 3, 1890, Edinburgh pathologist William Russell (1852-1940), gave an address to the Pathological Society of London outlining his microscopic findings of "a characteristic organism of cancer" that he observed in carbol fushsine-stained tissue sections from all forms of cancer that he studied, as well as in certain cases of tuberculosis, syphilis and skin ulceration. The parasite was seen within tissue cells (intracellular) and outside the cells (extracellular). Its size ranged from barely visible, up to "half again as large as a red blood corpuscle." (A red blood cell or erythrocyte is about 7 microns in diameter.) The largest size of the organism suggested a fungal or yeast-like parasite. Russell called the forms "fuchsine bodies" because of their bluish-red staining qualities [1]. Pathologist Harvey Gaylord in a 1901 report titled ‘The protozoon of cancer’, in the American Journal of the Medical Sciences, confirmed Russell’s research by finding similar bodies in every cancer he examined. The largest spherical bodies attained the amazing size of 50 microns in diameter, roughly seven times the diameter of a red blood cell! The common small round coccoid forms appeared the size of ordinary staphylococci [2]. By the turn of the 20th century most doctors concluded Russell bodies were merely the result of cellular degeneration or breakdown of one kind or another; and the idea of a cancer germ was discredited. However, Russell bodies became well known to pathologists, who generally regard them as non-microbial intracellular bodies primarily found in plasma cells (a type of white blood cell/leukocyte). More details on Russell and the ensuing history of cancer microbe research can be found in Cantwell’s 'The return of the cancer parasite' and ‘The Russell body: the forgotten clue to the bacterial origin of cancer, ' posted on www.rense.com. Russell’s original 1890 paper is posted online (Google: brmedj04652-0016). Figures 1 and 2 show examples of Russell bodies in an enlarged lymph node in vivo in an AIDS case.
Figure
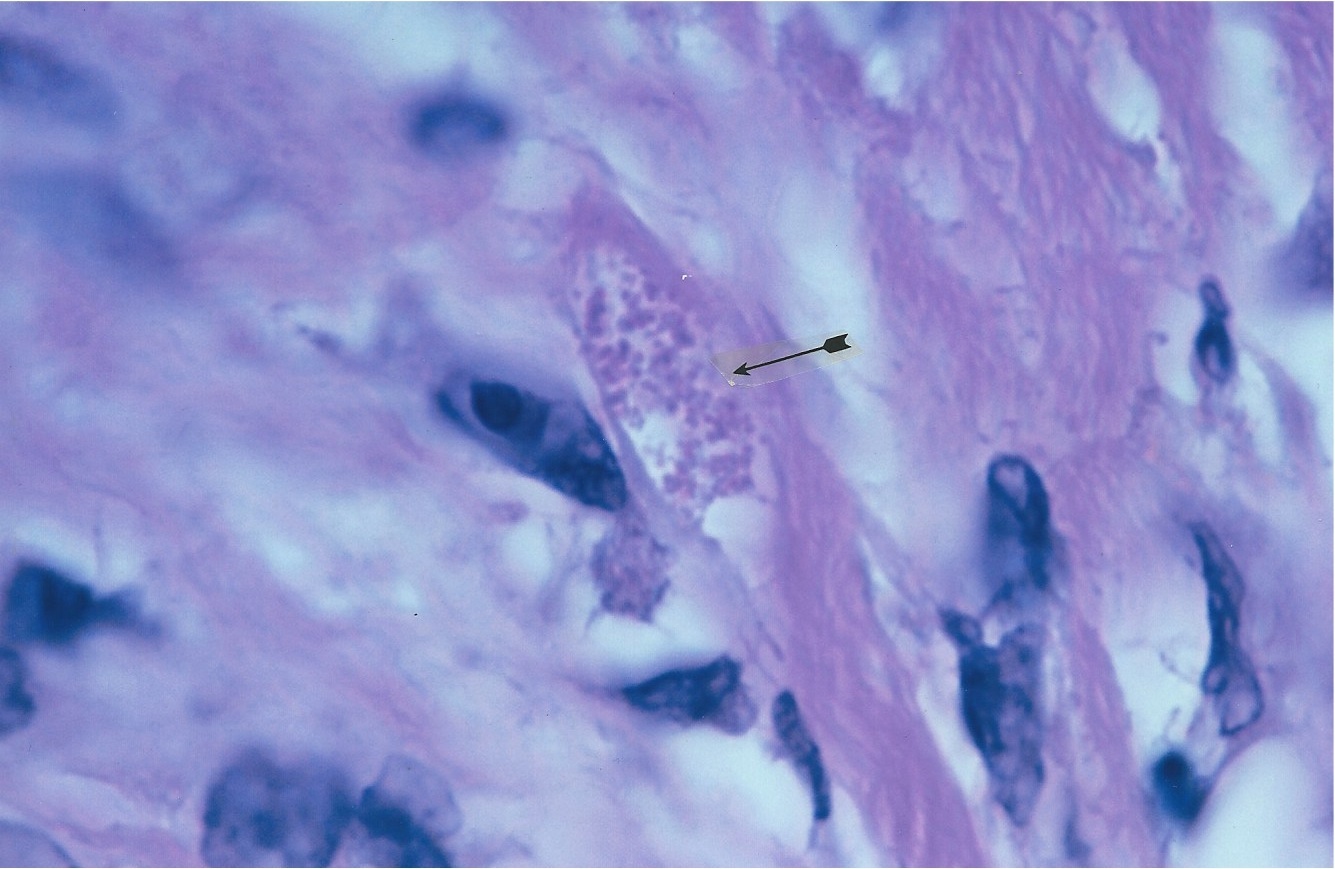
1. Enlarged lymph node from an AIDS case reported as “benign lymph node
hyperplasia”. The straight arrow points to a
Figure 2. Same lymph node as Figure 1. Arrow points to a collection of large, balloon-like forms of Russell bodies. Fite (acid-fast) stain, x1000
Virginia Livingston and the “life cycle” of the cancer microbe The most outspoken and controversial physician promoting the bacterial cause of cancer was my mentor Virginia Wuerthele-Caspe Livingston (1906-1990). If it were not for my scleroderma research, it is unlikely that I would have crossed paths with her. We both independently discovered CWD acid-fast mycobacteria in scleroderma, which led to a lifelong friendship. Her discovery in 1947 segued into cancer bacteria research [3]. My discovery in the mid-1960s led to a search for similar CWD forms of bacteria in a variety of cancerous and non-cancerous skin diseases, and eventually led into AIDS research [4-6]. In ‘The microscopy of micro-organisms associated with neoplasms’, published in 1948 in The New York Microscopical Bulletin, Livingston’s colleague Roy Allen describes how the small forms of the cancer microbe can be identified in vivo by use of the acid-fast stain [7]. This incorporates a basic fuchsine stain, similar to what Russell used. The hematoxylin-eosin stain (H&E), used routinely by pathologists, is not satisfactory for a critical demonstration of these forms, nor is the Gram stain, traditionally used to stain bacteria. The microbe can be rod-shaped or coccus shaped, and stains acid-fast (red) or non-acid-fast (blue) or variably acid-fast (purple). The much more common non-acid-fast coccal forms appear as single, double, or as densely packed round forms. The cocci are found intra- and extracellularly, and vary in size from 1 micron down to the smallest size the eye can detect, about 0.2 micron. Maximum proliferation occurs when the microbe is intracellular. Sooner or later the organisms break out from the confines of the cell and migrate to surrounding tissues. Multiplication may also take place outside of the cells, especially when collagenous (connective) tissue is present. The organisms show a decided preference for collagen. Allen emphasizes that “the organisms are acid-fast in some phase of their life cycle, thus indicating a close affinity with the mycobacteria. It is possible that these organisms have been seen and noted by practically every pathologist, but interpreted as eosinophilic (pink-staining) granules, hence their true nature not recognized. Only the Ziehl-Neelsen (acid-fast) technic reveals the difference.” In 1950 Virginia and her associates presented their cancer research in the American Journal of Medical Sciences in a paper entitled ‘Cultural properties and pathogenicity of certain microorganisms obtained from various proliferative and neoplastic diseases.’ A classic description of the microbe was provided. “These organisms, which appear primarily as small acid-fast granules in young cultures and which tend to become non-acid-fast in the larger forms present in old cultures, may exhibit a number of types, such as: a) minute filterable granules beyond the limits of visibility of the light microscope; b) larger granules approximately the size of ordinary cocci, readily seen with the light microscope; c) still larger globoidal forms; d) rod-like forms with irregular staining; and e) occasionally globoidal forms which appear to undergo polar budding” [8]. The virus-sized forms were photographed in electron microscope preparations. Livingston obtained these submicroscopic forms by filtering bacterial cultures obtained from cancer tumors. In order to prove that bacteria originate from these filtered (bacteria-free) cultures, she prepared multiple vials of bacteria-free filtrates from a single cancer microbe culture. Every few days, one of the bottles was opened and the filtrate examined. With the proper growth media and the passage of time, cancer bacteria were seen to reappear in the filtered bacteria-free fluid. This was proof to her that cancer bacteria originated from submicroscopic virus-like forms. Livingston never wavered in her belief in a cancer microbe, but the medical establishment ignored her cancer germ — a microbe that violated the established laws of microbiology and did not exist [9]. Figure 3 shows the cancer germ appearing as intra- and extracellular coccoid forms in an acid-fast stained tissue section of AIDS-related Kaposi’s sarcoma of the skin.
Figure 3. AIDS-related Kaposi’s sarcoma of the skin showing extracellular coccoid forms in the dermis. Fite (acid-fast) stain, x1000.
The "life cycle" of CWD mycobacteria in culture (in vitro) The smallest object visible in the light microscope is about 0.2 micron (a micron is 1/1000 of a meter). This is the size of the "granule" forms of CWD mycobacteria. The granules can enlarge to the size of round, staphylococcal-sized forms (0.5-1.5 microns). These coccoid forms in vitro can further enlarge to produce "Large Body” forms which range in size from 1 micron up to the amazing size of 150 microns [10]. This is 20 times the diameter of a red blood cell! These reproductive Large Bodies can also contain the tiny granules which escape their confines and re-initiate the transformative "life cycle." Russell hinted at this process in 1890 when he wrote: “A large fuchsine body gives either off or out a small granular body." By use of the Gram stain (used to stain bacteria), "smaller spores (i.e. coccoid forms) can perhaps be seen than by other staining methods and some appearances are exceedingly suggestive of a parent body having vomited out a number of minute spores. The smallest forms of his "parasite" did not stain the same way as his larger fuchsine bodies. "Our method of staining acts best when the organism is at a certain stage of growth, and that the smallest spores and degenerating larger individuals either do not stain differentially or they stain purple from a combination of the two colours used" [1]. Recently, Velayati et al. studied stress induced changes (via oxygen depletion) in lab experiments with typical acid-fast rod (bacillary) forms of Mycobacterium tuberculosis measuring 1.5 to 3 microns in size. The rods adapted to the stress by the formation of smaller ovoid cells, a budding type of cell division, the production of “spore-like cells” (around 0.5 micron in size), and filterable (virus-like) non-acid-fast forms. The investigators hypothesize these transformed forms are more virulent and more resistant against the body’s immune system [11].
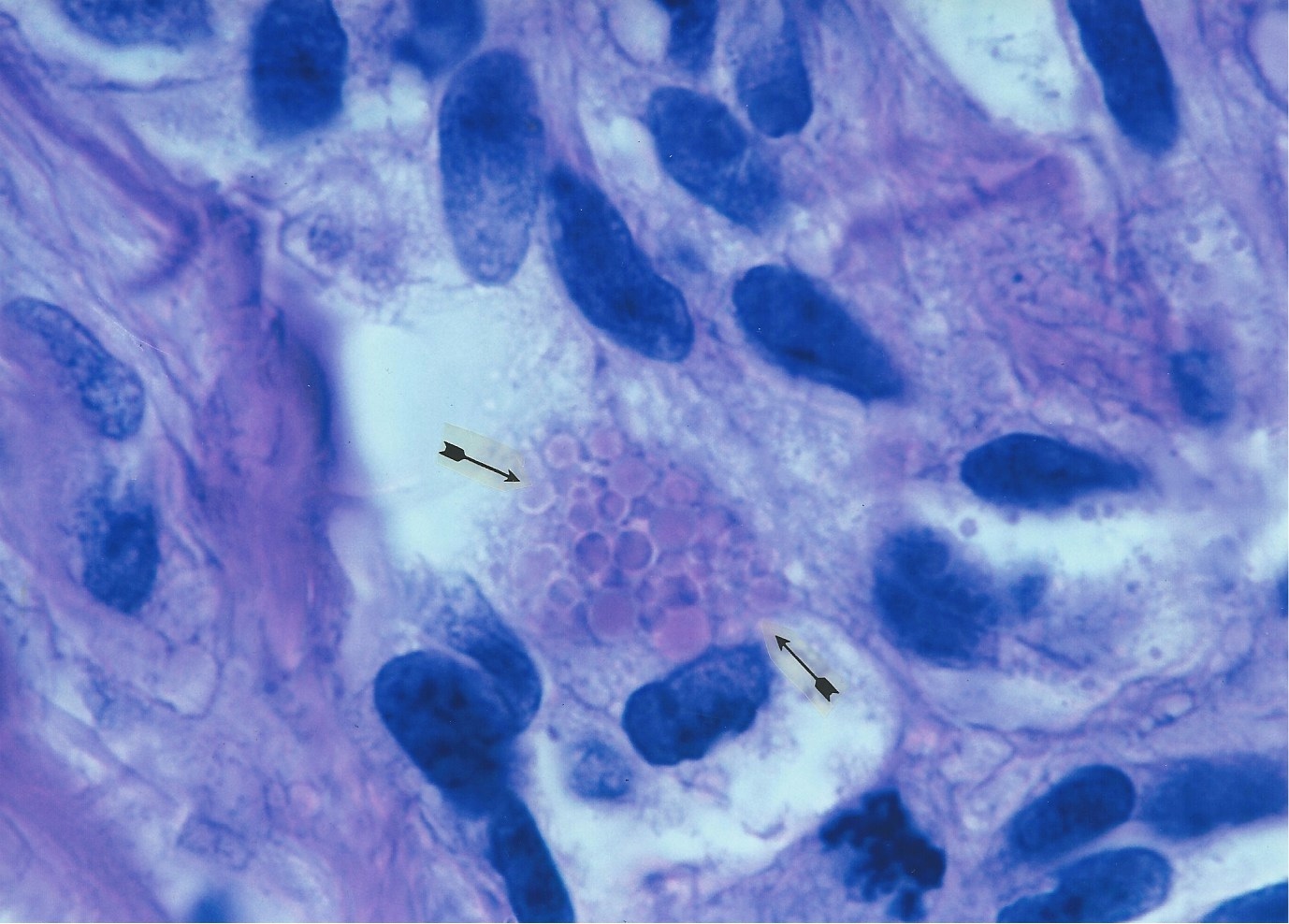
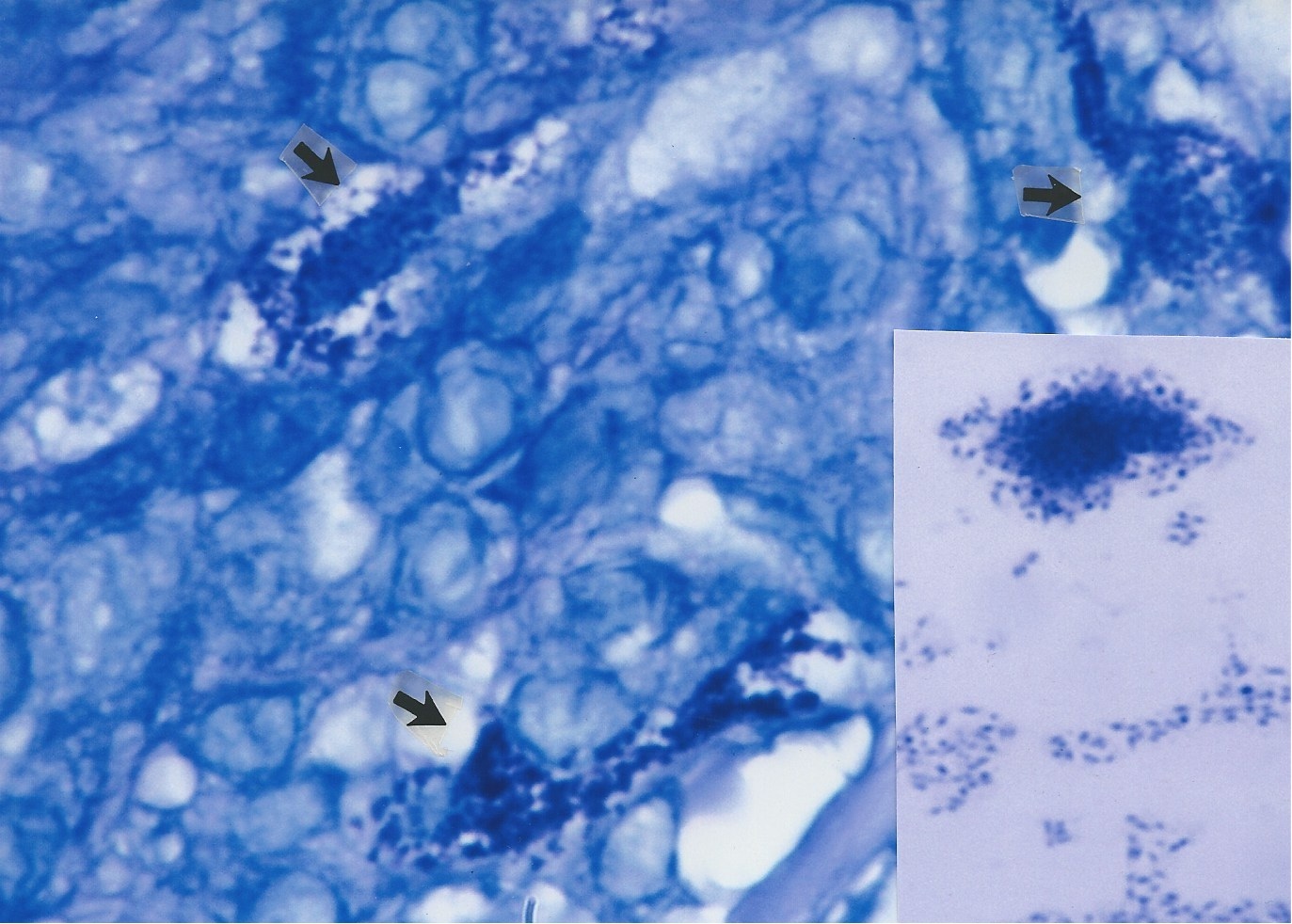
African “eosinophilic bodies” in Kaposi’s sarcoma and their originKaposi’s sarcoma (KS) was first reported by Moriz Kaposi in 1872 in Vienna. Until the AIDS epidemic it was a rare disease in America, but it has long been a common tumor in Central Africa. Eosinophilic bodies were first described by Murray and Lothe in endemic African KS cases in 1961 [12]. They consist of intra- and extracellular pinkstained spherical globules found in and around the cancerous “spindle cells” characteristic of KS tumors. The bodies varied in size from 10 microns in diameter down to 1 micron. Lothe noted the smallest size of the bodies was 1 micron or less, the size of staphylococci [13]. Lee, in 1968, thought the bodies represented disintegration of cells and noted the similarity to Russell bodies [14]. These bodies were originally thought to be specific to KS, but they were later also observed in European cases of “classic KS” not associated with HIV [15]. They have been observed in other forms of cancer as well [16]. Their origin is controversial and unresolved. Fukunaga and Silverberg think these bodies represent digested red blood cells [17], others suggest they are “lysosomal degenerative bodies” with glycoprotein content [15] or disturbed secretions of immunoglobulins [18]. Figures 4 and 5 show African eosinophilic bodies in AIDS-related Kaposi’s sarcoma of the skin.
Figure
4. AIDS-related Kaposi’s sarcoma of the skin. Arrows point to a collection
of tightly-packed, variable sized, transparent,
Figure
5. AIDS-related Kaposi’s sarcoma of the skin. In center a nest of pink-stained
coccoid forms wedged between
Eosinophilic Bodies and the question of “hyaline”The Russell body is currently defined as representing intracellular plasma cell immunoglobulins. (Immunoglobulins are glycoproteins secreted by plasma cells and that function as antibodies.) The term “eosinophilic body,” as used by pathologists (both in KS and other diseases), is difficult to define, at least for me. The word “eosinophilic” is merely descriptive and refers to the pink-staining color of cells and tissue forms when viewed with the pathologist’s routine H&E stain. It is not clear from various reports whether eosinophilic bodies are similar or identical or unrelated to Russell bodies. Some reported eosinophilic bodies observed in cancerous and non-cancerous diseases fit Russell’s description of perfectly round spheres. Others do not. To further confuse the issue, subsequent researchers studying KS eosinophilic bodies call them “hyaline globules.” This is unfortunate because hyaline per se is not a biochemical component of the eosinophilic bodies in KS nor the cells that contain them. Russell bodies, eosinophilic bodies, and so-called hyaline globules have all been found in various cancerous and non-cancerous diseases [19]. In histopathology the word “hyaline” is only a generic term applied to any homogenous, glassy, pink body when stained with H & E. Papadimitriou et al. conclude the presence of hyaline globules reflects stages of cell death [16]. How all these ubiquitous globules relate to one another (if at all) is unclear. Pathologists use the word “hyalinization” to refer to a change in collagen fibers (the connective tissue substance) in which the fibers fuse together to form a glassy, eosinophilic material. For example, infection with acid-fast mycobacteria can lead to hyalinization of the tissue; and in scleroderma in which TB-like CWD mycobacteria were found by Livingston and Cantwell, the connective tissue is hyalinized [3-6]. Russell also used the terms “hyaline-like structure” and “hyaline bodies” in connection with his “fuchsine bodies.” He was also unsure as to the precise meaning of hyaline. When I asked a pathologist to define Russell and eosinophilic bodies, he opined in an e-mail: “Russell bodies are intracellular accumulations of immunoglobulins; they are eosinophilic. But not all eosinophilic bodies are Russell bodies or immunoglobulins in clusters.” For more on hyaline, Google “hyaline change” at www.Pathopedia-India.com Like Russell and eosinophilic bodies, the true origin and significance of hyaline globules is unknown.
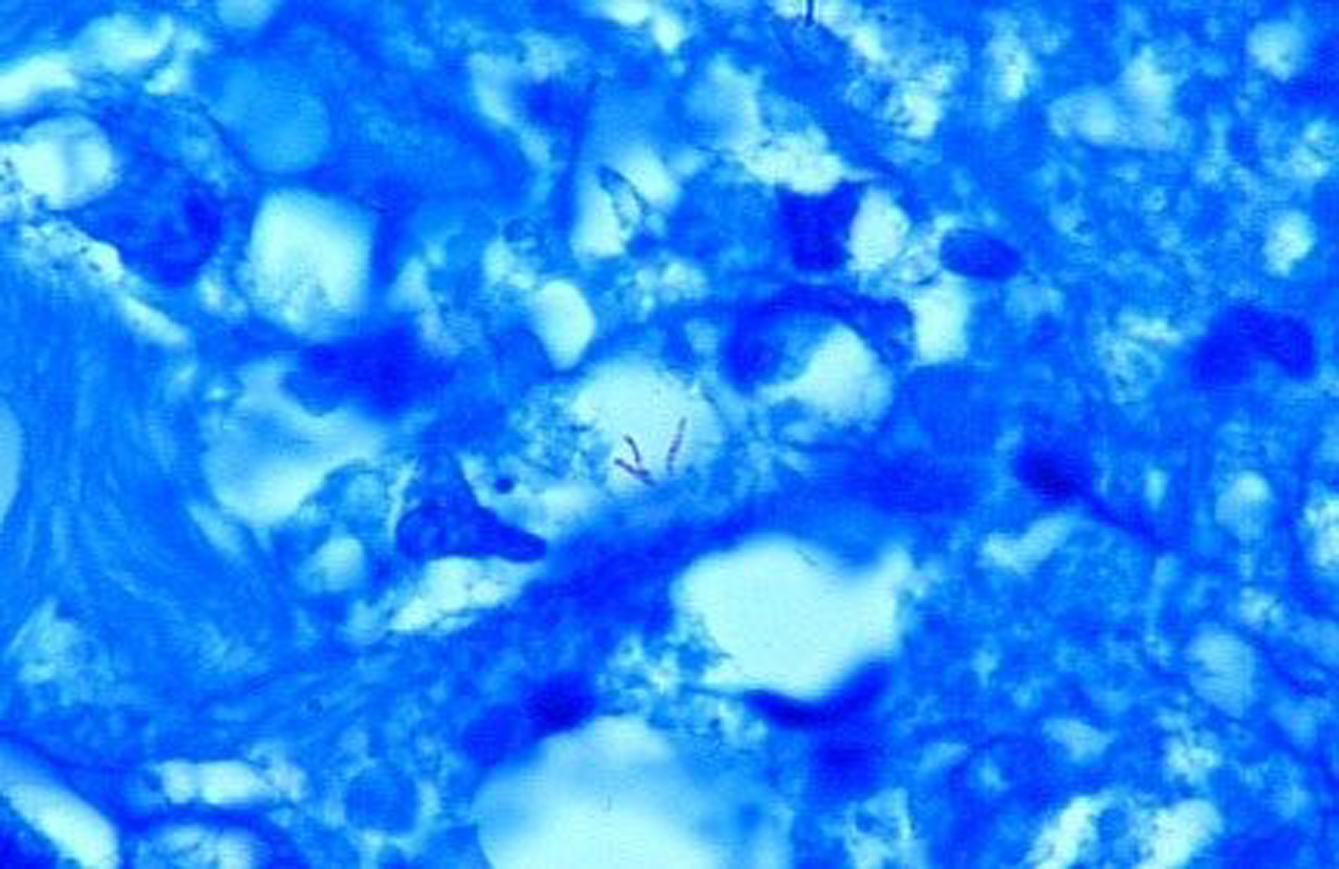
Kaposi’s sarcoma and mycobacteria Over the past two decades it is generally accepted that the human herpes virus-8 is the cause of KS. However, the role of bacteria in KS has never been seriously investigated, probably because bacteria are assumed to be absent or of no etiologic importance in this disease. During my years of microscopic study of skin diseases I infrequently observed Russell bodies and similar bodies that I eventually suspected were actually “Large Bodies” of CWD bacterial origin. These large forms were seen in vivo in scleroderma associated with Mycobacterium fortuitum infection, and in pseudoscleroderma, panniculitis, sarcoidosis, lupus erythematosus, and other diseases [6, 20]. In the early years of the AIDS and KS epidemic Cantwell et al. reported in a series of papers that CWD forms of bacteria were found in both classic and AIDS-related KS, as well as in the enlarged lymph nodes in the early stages of HIV infection, and throughout the body at autopsy of AIDS and KS patients [21-26]. In addition, mycobacterial infection with Mycobacterium avium-intracellulare was reported in a case of AIDS-associated “immunoblastic sarcoma” [27]. In all these cases, intra- and extracellular bacteria were observed in tissue sections stained for acid-fast bacteria. See the examples in Figures 6-9. Prior to the 1984 discovery of HIV as “the sole cause of AIDS”, I had suggested CWD acid-fast mycobacteria were implicated in the cause. Broxmeyer has also hypothesized mycobacterial infection as a primary and overlooked cause of AIDS and cancer [28, 29]; and Mattman has reviewed the literature pertaining to CWD bacteria in the etiology of cancer [30]. According to the World Health Organization, about one-third of the world’s population has latent tuberculosis (TB), caused by Mycobacterium tuberculosis. Approximately 40% of men and women will also be diagnosed with cancer at some point in their lifetime. The incidence of latent TB and non-tuberculous mycobacteria in AIDS patients is not known. However, TB is the leading killer of HIV-positive people, causing one-fourth of all HIV-related deaths. Occult bone marrow infection with Mycobacterium avium-intracellulare was noted very early in the American AIDS epidemic. In 1982, eight of the first nine autopsied AIDS cases at UCLA died of Mycobacterium avium infection (31) . It is now undeniable that mycobacteria are pleomorphic and exist in more than one morphologic form [11]. However, in clinical pathology there is only one form currently accepted as a bona-fide form of mycobacteria in vivo, and that is the acid-fast (red stained) rod form (bacillus) . The other non-acid-fast pleomorphic forms are simply not generally recognized or reported by pathologists or dermatopathologists. On rare but significant occasions, researchers have reported the co-existence of KS and M. tuberculosis [32] or other mycobacterial infection within the same biopsy specimen [33, 34]. M. tuberculosis and non-tuberculosis mycobacteria can also produce, albeit rarely, a “pseudo tumor” closely resembling KS. Such cases occur primarily in immunosuppressed patients with or without HIV infection. [35, 36].
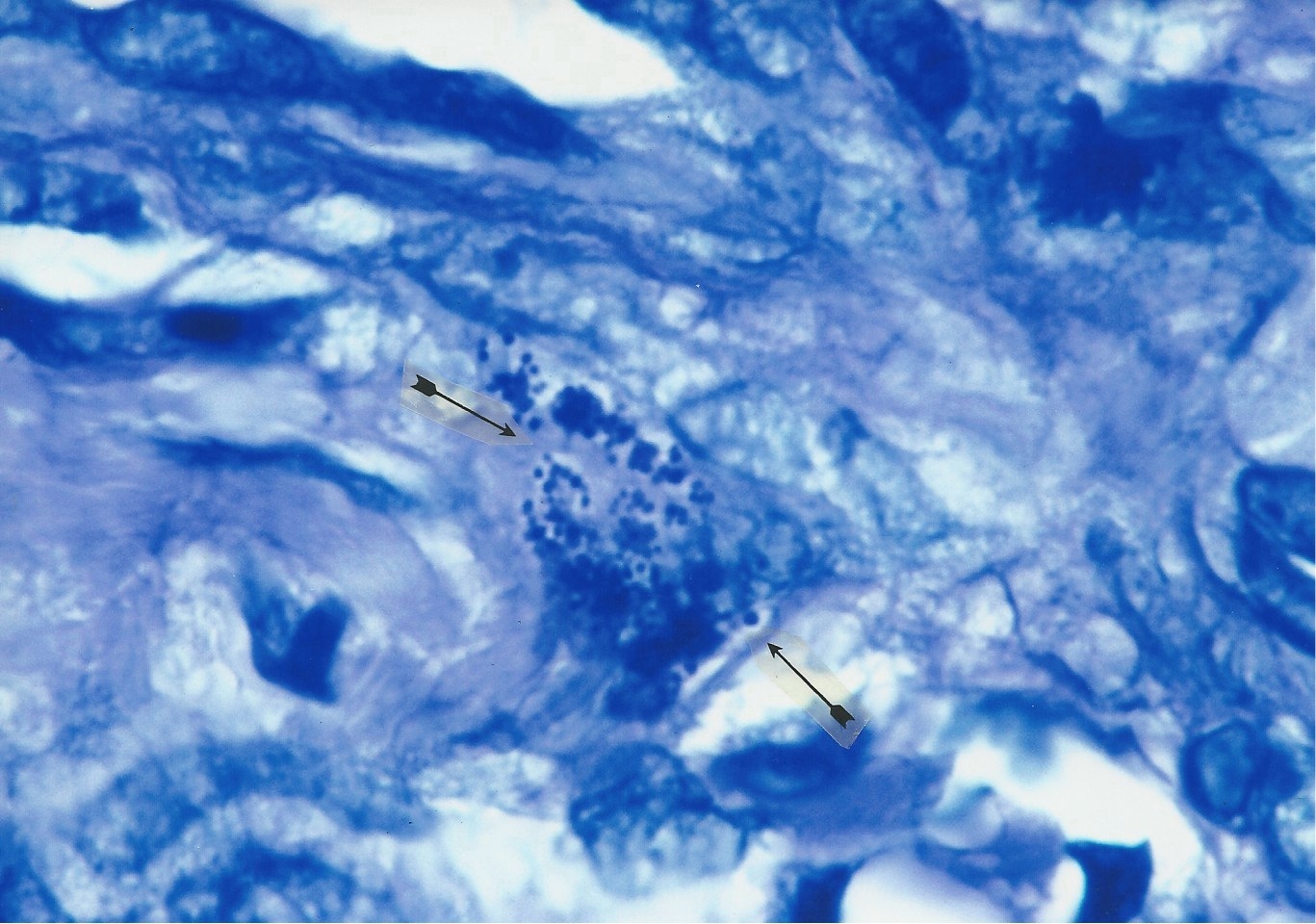
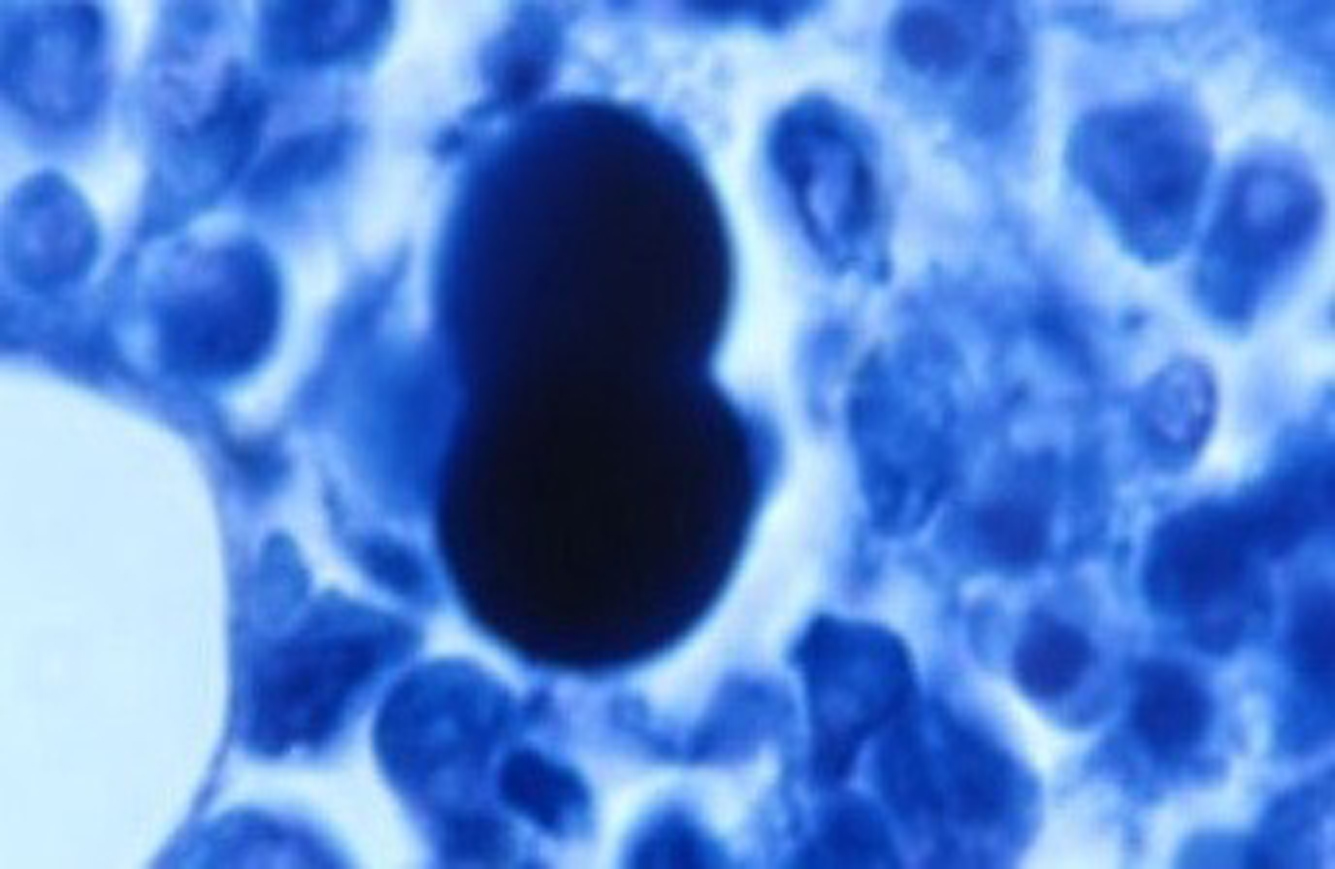
Figure
6. Bone marrow section taken from autopsy of a fatal case of AIDS. A
Giant Large Body appears to
Figure
7. Unstained transparent, large balloon-like forms and related small
coccoid growth forms of
Figure
8. Classic (non-HIV-related) Kaposi’s sarcoma. Arrows point to three
areas of coccoid forms and tinier barely visible granules
Figure
9. AIDS-related Kaposi’s sarcoma of the skin showing intra- and extracellular
coccoid forms. Giemsa stain, x1000.
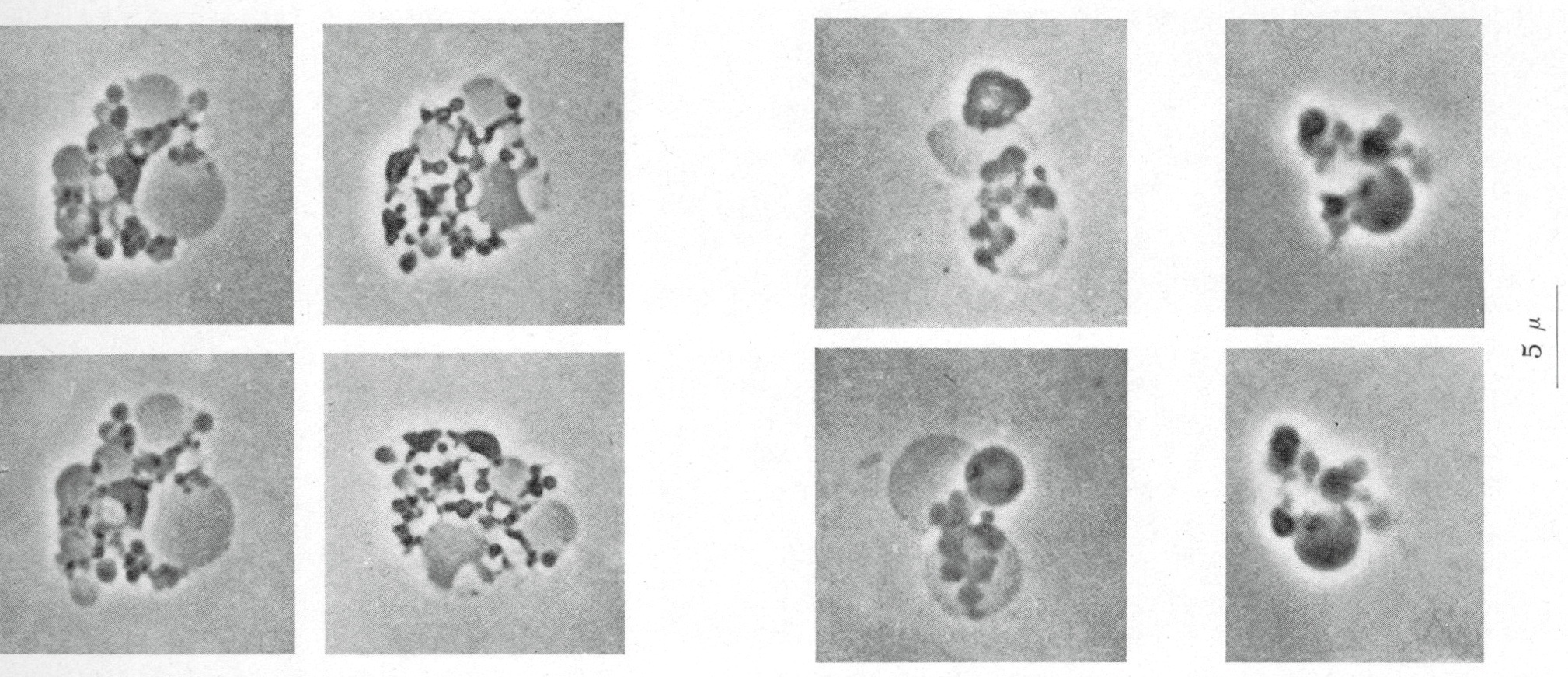
Is Kaposi’s sarcoma caused by CWD mycobacteria?The idea that KS is caused by bacteria is admittedly heretical. The essential tissue stain for detection of the cancer microbe is the acid-fast stain. However, it is rare to find typical acid-fast (red-stained) rod-shaped bacillary forms of mycobacteria in KS, but such cases have been reported, as mentioned above. Only once could I detect acid-fast rod forms in cancer, in a reported case of AIDS-associated immunoblastic sarcoma [27]. However, the acid-fast stain will usually reveal the non-acid-fast or partially acid-fast coccoid and granular forms of CWD bacteria that represent the smaller forms of the cancer germ in vivo. These coccoid forms may morph into Large Bodies, which appear similar to Russell bodies. The numerous coccoid forms in KS, particularly in AIDS-related cases, might be misinterpreted as mast cell granules, but such granules do not attain the size of staphylococci, still larger “globoid forms” and Large Bodies [8]. “Large Body” forms of CWD bacteria in vivo and in vitroWhen we think of bacteria we generally think of a coccus or a rod form. We are taught that common bacteria simply divide in half via “binary fission” to produce an offspring that looks similar. It is difficult (perhaps preposterous) to think of a bacterium that is globular and transparent and 20 times the diameter of a red blood cell! In the late 1970s when I first became aware of the research of microbiologist Lida Mattman, I was amazed to discover the wide variation of forms that CWD mycobacteria express as part of their “life cycle” in vitro [10]. Figure 10 illustrates a Giant Large Body which appears to be budding (like a fungus) in a bone marrow tissue section in vivo at autopsy of a fatal AIDS case [25]. I was also fortunate to be presented with a monograph, “L-forms of mycobacteria and chronic nephritis,” by the late Conrado Xalabarder of Barcelona that clearly shows the association and back-and-forth transformation between perfectly round, large, transparent, balloon-like Large Bodies and coccoid-sized growth forms of M. tuberculosis, observed and photographed in phase contrast microscopic preparations [37]. A few of these pleomorphic mycobacterial forms are illustrated in Figure 11. For further study of CWD bacteria, I recommend Lida Mattman’s seminal text Cell Wall Deficient Forms, and Gerald Domingue’s (Ed) Cell Wall-Deficient Bacteria.
Figure
10. AIDS-related Kaposi’s sarcoma of the skin. Arrows point to intra-and
extracellular coccoid forms in the dermis. Fite (acid-fast) stain, x1000.
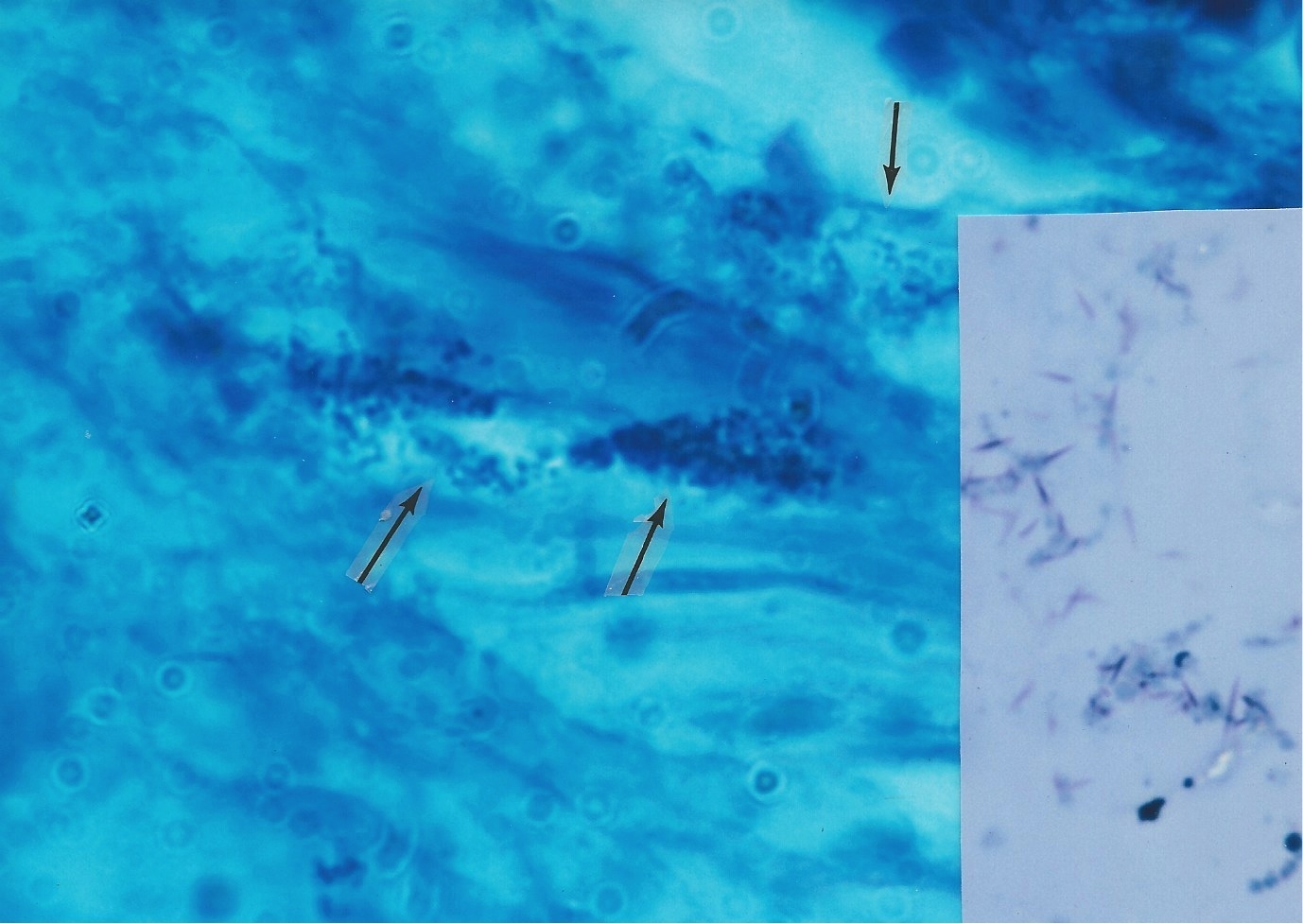
Figure
11. Skin tumor of AIDS-related immunoblastic sarcoma showing three very
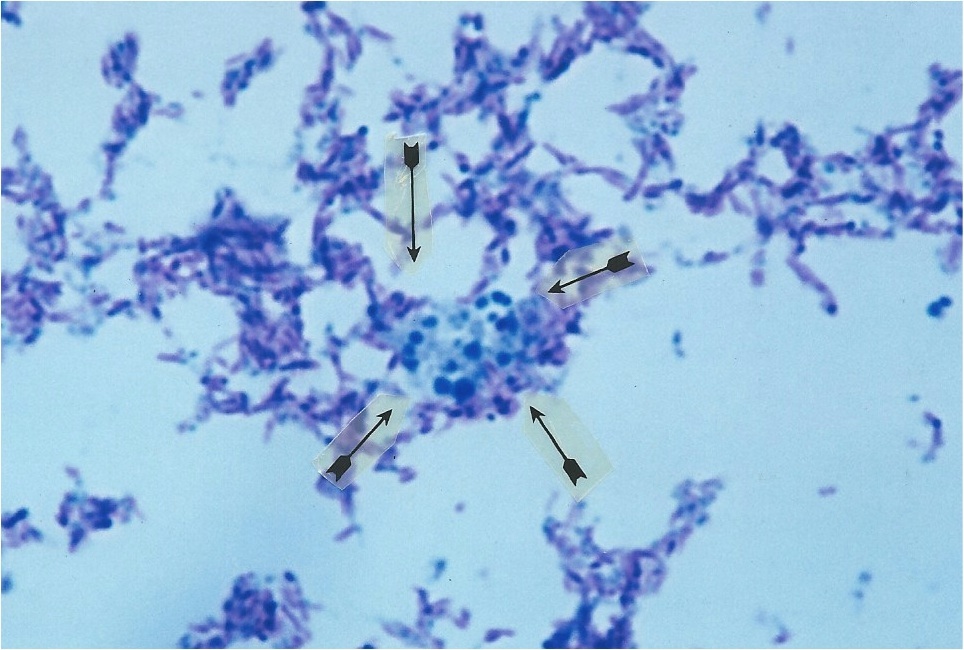
rare acid-fast rod forms of mycobacteria in the dermis. Are Russell bodies CWD mycobacteria in “Large Body” form?In 1980 Cantwell proposed that Large Bodies of microbial origin could be found in vivo in scleroderma and pseudoscleroderma [20]. Large Bodies in vitro, with their perfectly round transparent spheres, are strikingly similar in size and shape to Russell bodies, eosinophilic bodies, and hyaline globules in vivo. The various theories of origin for eosinophilic bodies and hyaline globules (such as representing accumulations of immunoglobulins, fragmented red blood cell elements, various cellular degenerations, programmed cell death, plasma cell inclusions, and glycoprotein) are compatible with a proposed bacterial origin . For example, chronic intracellular bacterial infection provokes a plasma cell and immune response with the production of immunoglobulins. CWD bacteria are intracellular pathogens with an affinity for red blood cells [38-40]. Mycobacterial infection can induce cell damage and initiate programmed cell death (apoptosis) [41]. CWD bacteria have a predilection for particular cells (leukocytes, erythrocytes, endothelial, epithelial) and collagenous tissues. These cells and tissues provide constituents which are required for the maintenance and survival of CWD mycobacteria [42]. Bacteria contain and synthesize glycoproteins [43]. In this regard, the Livingstons reported the cancer microbe produces a pregnancy-type hormone called human choriogonadotropin hormone (HCG) which is a glycoprotein [44]. Other little-known microscopic findings also suggest occult mycobacterial infection in vivo. The periodic acid Schiff (PAS) tissue stain is commonly used to detect fungus infection. Russell and eosinophilic bodies test “positive” with the PAS stain, and so do intracellular mycobacteria [45, 46]. KS eosinophilic bodies autofluorescence under ultraviolet illumination [45]; mycobacteria also autofluorescence [47]. KS eosinophilic bodies have been described as “refractile”; and mycobacteria have this quality as well [48]. Recently, Nadya Markova, in ‘Cell wall deficiency in mycobacteria: Latency and persistence’ (available online via open access), emphasizes that survival of mycobacteria in vivo involves conversion to CWD forms, which propagate in tissue macrophages and escape destruction by the immune system. In rats injected with M. tuberculosis, Large Bodies were observed within vacuoles in the macrophages [49]. Russell’s Figure 1 illustrates a “vacuole containing two organisms” [1]. Markova notes that coccoid forms of mycobacteria may be indistinguishable from ordinary staphylococci or other coccus-shaped bacteria. When cultured from clinical specimens, exclusively CWD coccoid forms of M. tuberculosis can easily go unrecognized and be mistaken for ordinary lab contaminants. She herself was fooled when a ‘staphylococcus’ was cultured from one of her TB-infected rats. However, genetic testing proved the coccus was, in reality, a CWD form of M. tuberculosis! If one carefully observes smears made from culture of the non-tuberculous Mycobacterium avium-intracellulare, it is evident that this microbe is pleomorphic. Easily overlooked are the non-acid-fast, blue-stained coccoid forms and the still smaller, barely visible light blue granular forms seen in Figure 12. If a culture of CWD mycobacteria consists predominantly of CWD coccal forms, it could easily be misinterpreted as common staphylococci or other micrococci and dismissed as a “contaminant.” For that reason, all smears showing coccal and cocco-bacillary forms cultured from cancer should be subjected to an acid-fast stain. Livingston always stressed the cancer microbe could be identified by its intermittent acid fastness. Occasionally, smears reveal acid-fast “spicules” emanating from the cocci, as noted in the streptococci cultured from KS (see, the inset of Figure 8). The exact significance is not clear, but the spicule phenomenon was reported by Livingston and Alexander-Jackson in some of their cancer isolates, and may serve as an additional clue to a possible mycobacterial origin of the lab culture [50]. These unprecedented microbiologic observations in mycobacterial research could partially explain the century-old difficulty cancer microbe researchers have encountered in trying to convince colleagues that pleomorphic mycobacteria posing as common cocci are implicated in the etiology of cancer. That is why recognizing pleomorphic forms of mycobacteria in tissue is so important. Cocci cultured in vitro from cancer are almost always regarded as insignificant, but the pathologic finding of mycobacteria in vivo is always significant.
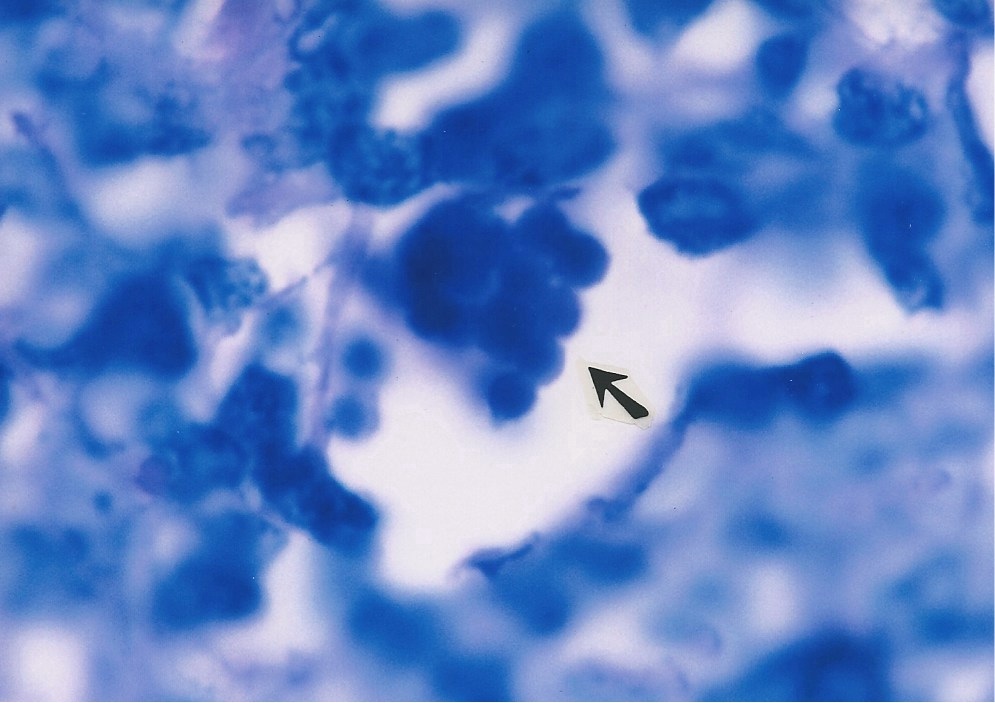
Figure
12. Smear of pleomorphic Mycobacterium avium-intracellulare cultured
from immunoblastic sarcoma
The cancer germ in the 21st century Was it a scientific blunder to dismiss bacteria as a cause of cancer, and at a time when bacteriology was in its infancy and CWD bacteria were unknown? Some bacteria, like the 1982 discovery of pleomorphic Helicobacteria that cause stomach ulcers, were hidden from the pathologist’s view for a century. A proper stain finally allowed their detection in vivo, thereby overthrowing medical dogma claiming bacteria could never live and thrive in the acid environment of the stomach. Was Russell correct in his discovery of a cancer germ? It is now recognized that 90% of our body cells are microbial cells, primarily bacterial cells. We harbor 100 trillion bacteria of various types, mostly unstudied species. Yet bacteria are rarely, if ever, mentioned in the reports of pathologists who examine cancerous tissue. Could microbes be hiding in cancer tissue in an unrecognized form — or remain undetected because a proper stain is not used? Hopefully, this report will stimulate others to continue the search for a cancer microbe. Perhaps in the form of a Russell body, an eosinophilic body, a hyaline globule, a CWD Large Body, a granule, a coccoid form, a spore, a fungus-like form, a “new virus,” or a new tissue form waiting to be discovered. [Alan Cantwell is a retired dermatologist. He is the author of The Cancer Microbe and Four Women Against Cancer, available from Amazon.com. Abstracts of his scientific papers can be found on the www.PubMed.gov website.]
References: 1.Russell W (1890) An address on a characteristic organism of cancer. Br Med J 2:1356-1360 2. Gaylord HR (1901) The protozoon of cancer. Amer J Med Sci; 121:501-539 3. Wuerthele-Caspe (Livingston) V, Brodkin E, Mermod C (1947) Etiology of scleroderma. preliminary clinical report. J Med Soc New Jersey 44:256-259 4. Cantwell AR, Craggs E, Wilson JW, et al (1968) Acid-fast bacteria as a possible cause of scleroderma. Dermatologica 136:141-150 5. Cantwell AR (1984) Histologic observations of pleomorphic, variably acid-fast bacteria in scleroderma, morphea and lichen sclerosus et atrphicus. Int J Dermatol 23:45-52 6. Cantwell A (1982) Variably acid-fast cell wall-deficient bacteria as a possible cause of dermatologic disease. In: Cell Wall Deficient Bacteria: Basic Principles and Clinical Significance. Domingue, GJ (ed.). pp. 321-360. Addison Wesley Publishing Co., Reading, PA 7. Allen RM (1948) The microscopy of micro-organisms associated with neoplasms. New York Micro Soc Bull 2:19-26 8. Wuerthele Caspe-Livingston V, Alexander-Jackson E, Anderson JA, et al (1950) Cultural properties and pathogenicity of certain microorganisms obtained from various proliferative and neoplastic diseases. Amer J Med Sci 220:628-646 9. Anonymous (1990) Unproven methods of cancer management—Livingston-Wheeler therapy. CA Cancer J Clin 40:103-108 10. Mattman L, Tunstall L, Mathews W, et al (1960) L variation in mycobacteria. Am Rev Resp Dis 82:202-211 11. Velayati AA, Farma P, Masjuedi MR et al (2011) Sequential adaptation in latent tuberculosis bacilli: observation by atomic force microscopy (AFM), Int J Clin Exp Med 4:193-9 12. Murray JF and Lothe F (1962) The histopathology of Kaposi’s sarcoma. Acta Un Int Cancer 18: 413-428 13. Lothe F (1963) Kaposi’s sarcoma in Uganda Africans. Acta Path Micrbiol Scand (Supple) 161:1-70 14. Lee FD (1968) A comparative study of Kaposi’s sarcoma and granuloma pyogenicum in Uganda. J Clin Pathol 21:199-128 15. Massarelli G, Scott CA, Mura A et al (1989) Hyaline bodies in Kaposi’s sarcoma: an immunocytochemical and ultrastructural study. Appl Pathol 7:26-33 16. Papadimitriou JC, Drachenberg CB, Brenner DS et al (2000) “Thanatosomes”: a unifying morphogenetic concept for tumor hyaline globules related to apoptosis. Human Pathol 31:1455-65 17. Fukunaga M, Silverberg SG (1991) Hyaline globules in Kaposi’s sarcoma: a light microscopic and immunohistochemical study. Mod Pathol 4:187-190 18. Munday WR, Kapur LH, Xu M, et al (2015) Russell body duodenitis with immunoglobulin kappa light restriction. World J Gastointest Endosc 7:73-6 19. Dikov DI, Auriault ML, Boivan JF, et al (2007) Hyaline globules (thanatosomes) in gastrointestinal epithelium: pathophysiologic correlations. Am J Clin Pathol 127: 792-9 20. Cantwell A (1980) Histologic forms resembling “large bodies” in scleroderma and pseudoscleroderma. Amer J Dermatopathol 2:273-276 21. Cantwell A (1981) Bacteriologic investigation and histologic observations of variably acid-fast bacteria in three cases of Kaposi’s sarcoma. Growth 45:79-89 22. Cantwell A, Lawson J (1981) Necroscopic findings of pleomorphic, variably acid-fast bacteria in a fatal case of Kaposi’s sarcoma. J Dermatol Surg Oncol 7:923-930 23. Cantwell A (1982) Variably acid-fast bacteria in vivo in a case of reactive lymph node hyperplasia occurring in a young male homosexual. Growth 46:331-336 24. Cantwell A (1983) Kaposi's sarcoma and variably acid-fast bacteria in vivo in two homosexual men. Cutis 32:58-61,63-4, 68 25. Cantwell A (1983) Necroscopic findings of variably acid-fast bacteria in a fatal case of acquired immunodeficiency syndrome and Kaposi's sarcoma. Growth 47:129-34 26. Cantwell A, Rowe L (1985) African "eosinophilic bodies" in vivo in two American men with Kaposi's sarcoma and AIDS. J Dermatol Surg Oncol 11:408-12 27. Cantwell A (1986) Mycobacterium avium-intracellulare infection and immunoblastic sarcoma in a fatal case of AIDS. Growth 50:32-40 28. Broxmeyer L and Cantwell AR (2008) AIDS: “it’s the bacteria, stupid.” Med Hypothesis (2008) Med Hypothesis 71:741-8 29. Broxmeyer L (2004) Is cancer just an incurable infectious disease? Med Hypothesis 63:986-96 30. Mattman L (1993) Microbes and malignancy. In,: Mattman L, Cell wall deficient forms: Stealth pathogens. (Ed2), CRC Press, Boca Raton 31. Zakowski P, Fligiel S, Berlin GW et al (1982) Disseminated Mycobacterium avium-intracellulare infection in homosexual men dying of acquired immunodeficiency. JAMA 248:2980-2982 32. Croxson TS, Ebanks D, Mildvan D (1983) Atypical mycobacteria and Kaposi’s sarcoma in the same biopsy specimen. N Engl J Med 308:1476 33. Ramdial PK, Sing Y, Subrayan S et al (2010) Granulomas in acquired immunodeficiency syndromes-associated cutaneous Kaposi’s sarcoma: evidence for a role of Mycobacterium tuberculosis. J Cutan Path 37:827-3 34. Bodhireddy H, Rivas S, Sexhadri, T (2010) Coexistent Kaposi’s sarcoma and atypical mycobacterial infection involving lymph node: a case report and review of the literature. Indian J Pathol Microbiol 53:805-807 35. Logani S, Lucas DR, Cheng JD, et al. (1999) Spindle cell tumors associated with mycobacteria in lymph nodes of HIV-positive patients: 'Kaposi sarcoma with mycobacteria' and 'mycobacterial pseudotumor'. Am J Surg Pathol 23: 656-61 36. Basilio-de-Oliviera C, Eyer-Silva WA, Valle HA et al. (2001) Mycobacterial spindle cell pseudotumor of the appendix vermiformis in a patient with AIDS. Braz J Infect Dis 5: 98-10 37.Xalabarder C (1970) L-forms of mycobacteria and chronic nephritis. Public Inst Antituberc Supple 7:7-83 (Barcelona) 38. Pohlod DJ, Mattman LH, Tunstall L (1972) Structures suggesting cell-wall-deficient forms detected in circulating erythrocytes by fluorochrome staining. Appl Microbiol 23:262 39. Domingue GJ, Schlegel JU (1977) Novel bacterial structures in human blood: cultural isolation. Infect Immun 15:621-7 40. Tedeschi GG, Bondi A, Paparelli M, et al. (1978) Electron microscopical evidence of the evolution of corynebacteria-like microorganisms within human erythrocytes. Experientia 34:458-60 41. Lancellotti M, Pereira RF, Cury GG, et al. (2009) Pathogenic and opportunistic respiratory bacteria-induced apoptosis. Braz J Infect Dis 13: 226-31 42. Domingue GJ, Schlegal JU, Woody HB (1976) Naked bacteria in human blood: a novel concept for etiology of certain kidney diseases. Microbia 2:1-29 43. Messner P (2004) Prokaryotic glycoproteins unexplored but important. J Bacteriol 186:2517-9 44. Livingston VWC and Livingston AM (1974) Some cultural, immunological and biochemical properties of Progenitor cryptocides. Trans N Y Acad Sci 36: 569-82 45. Senba M (1985) Autofluorescence of eosinophilic globules in Kaposi’s sarcoma. Arch Pathol Lab Med 109:703 46. Pappolla MA and Mehta VT (1984) PAS reaction stains phagocytosed atypical mycobacteria in paraffin sections. Arch Pathol Lab Med 108:372-3 47. Ghodbane R, Raoult D, Drancourt M (2014) Dramatic reduction of culture time of Mycobacterium tuberculosis. Sci Rep 28:4 48. Torlakovic E, Clayton F, Ames ED (1992) Refractile mycobacteria in Romanowsky-stained bone marrow smears. A comparison of acid-fast-stained tissue sections and Romanowsky-stained smears. Am J Clin Pathol 97:318-21 49. Nadya Markova (2012). Cell wall deficiency in mycobacteria: latency and persistence. In, Cardona PJ (Ed) Understanding Tuberculosis - Deciphering the Secret Life of the Bacilli. InTech:193-216 50.
Wuerthele-Caspe Livingston V, Alexander-Jackson (1970) A specific type
of organism cultivated from malignancy: Bacteriology and proposed classification.
Ann N Y Acad Sci 174:636-654 |
| Donate to Rense.com Support Free And Honest Journalism At Rense.com | Subscribe To RenseRadio! Enormous Online Archives, MP3s, Streaming Audio Files, Highest Quality Live Programs |