- Introduction
-
- During my career as a dermatologist, I was always interested
in searching for bacteria in skin biopsies in "acid-fast" stained
sections; and in finding bacteria in diseases in which bacteria were not
supposed to exist. For want of a better title, I refer to my controversial
body of published microscopic work as "cancer microbe" research.
-
- My first encounter with "pleomorphic" microbes
(bacteria having more than one form) began as a resident in the early 1960s,
when I discovered unusual tuberculosis-like acid-fast bacteria in vivo
(i.e. within the tissue) in four patients with panniculitis (an inflammation
of the fatty layer of the skin).
-
- Quite by accident, I also found acid-fast bacteria in
scleroderma in 1965. My dermatology professor J Walter Wilson, also a well-known
expert in fungal diseases, insisted I get a "negative" control
skin biopsy to counterbalance the repeated positive findings of acid-fast
bacteria I was encountering in panniculitis patients. He suggested a biopsy
from scleroderma, which would surely be negative for acid-fast bacteria,
as scleroderma is not considered a bacterial infection.
-
- When the technician in the tuberculosis (TB) laboratory
examined "smears" of the scleroderma biopsy, she detected typical
acid-fast stained bacilli, rod forms like those causing TB. After confirming
this remarkable finding, I assumed I could find acid-fast rod forms in
the patient's slides prepared by the pathologist. However, after countless
hours of study, I was only able to detect a few rods. (Fig 1.)
-
-

-
- Fig 1. SCLERODERMA. Extremely rare red-stained
stick-like bacillary rods in the deep dermis of a fatal case of scleroderma.
Fite-Faraco (acid-fast) stain, x1000
-
-
- I anxiously awaited the growth of a TB germ in culture,
but instead the scleroderma culture was a mix of non-acid-fast coccoid
forms and typical acid-fast rods (Fig 2.) But the pleomorphic culture
could not be precisely identified by the experts. From further biopsies
made several years later when the patient died of this disease, we were
able to grow and identify Mycobacterium fortuitum, an "atypical"
species of acid-fast mycobacteria. I wrote about the details of this case
in my book, "The Cancer Microbe." Early in my career I learned
that microbes could perform "tricks" I never learned about in
bacteriology in medical school; and sometimes the bacteria stumped the
experts.
-
-
-

-
- Fig 2. SCLERODERMA, PLEOMORPHIC ACID-FAST
BACTERIA. Culture from fatal case of systemic scleroderma showing two distinct
forms: non-acid-fast (blue stained) cocci and acid-fast (red-stained)
rod forms typical of mycobacteria. The precise identification of this bacterial
isolate could not be determined. Ziehl-Neelsen (acid-fast) stain, x1000
-
-
-
- Over the next few years I finally realized that finding
acid-fast bacilli in scleroderma biopsies was extremely difficult and time
consuming. However, variably acid-fast coccoid forms were fairly numerous,
even though these round, granular forms were basically ignored by my
colleagues. The "naked" coccoid forms seen in Fig 3 clustered
among the collagen fibers (without any surrounding cellular reaction)
is the common presentation of scleroderma bacteria in the dermis. Various
photos of the scleroderma microbe can be viewed in our paper entitled "Bacterial
infection as the cause of scleroderma," published at the www.joimr.org
website (Cantwell and Ganger, 2006).
-
-

-
- Fig 3. SCLERODERMA. A collection of "naked" blue-stained
coccoid forms in the deep dermis of the skin with no surrounding tissue
cells. This is the common appearance of the pleomorphic scleroderma microbe
in vivo. Fite-Faraco (acid-fast) stain, x1000
- Click here for larger image
-
-
-
- My panniculitis study (Cantwell et al) and my first unusual
scleroderma case (Cantwell and Wilson) were both reported in 1966. I never
dreamed these studies would provoke a lifelong interest in such work until
I met Virginia Livingston in 1968.
-
- The "cancer microbe" and the microbiology
of cancer
-
- My friend and mentor, the late Virginia Wuerthele_Caspe
Livingston MD, first discovered pleomorphic acid-fast bacteria in scleroderma
two decades earlier in 1947. Soon after, she and her colleagues began their
studies of similar bacteria in various forms of cancer (Wuerthele-Caspe
Livingston et al, 1950). In this research, she collaborated with microbiologist
Eleanor Alexander-Jackson, cell cytologist Irene Diller, and tuberculosis
icon Florence Seibert. I have written about these women, as well as other
cancer microbe researchers, in "Four Women Against Cancer."
-
- The search for a microscopically visible infectious agent
in cancer dates back to the late nineteenth century, when Scottish pathologist
William Russell reported "the parasite of cancer." Even though
his cancer microbe work was confirmed by a few other scientists, the idea
of a cancer-causing germ was totally rejected in the early years of the
last century for various reasons. Nevertheless, a few adventurous researchers
kept this research alive during the 1920s and 1930s, but their reports
were also dismissed or ignored by the medical establishment.
-
- Livingston was the first to show that the cancer microbe
could be detected in its many pleomorphic forms both in vivo and in vitro
by use of the acid-fast stain. This stain is traditionally used to detect
the acid-fast mycobacteria that cause TB (Mycobacterium tuberculosis)
and leprosy (M. leprae). Over the past half-century the number of recognized
mycobacterial species has greatly increased, and the list grows every
year. (For more details on the "acid-fast stain", as well as
"bacterial pleomorphism" and "atypical mycobacteria",
Google these key words.)
-
- The classic form of mycobacteria is a red-stained
acid-fast granular rod shaped bacillus. The pleomorphic cancer microbe
detected by Livingston and others, can be non-acid-fast (blue-stained),
weakly acid-fast (purple) or strongly acid-fast (red). Tissue and laboratory
findings indicate the cancer germ is most closely related to the acid-fast
mycobacteria. The smallest forms are filterable and virus-like; the largest
forms may resemble yeast or fungal-like cells; and the most frequently
encountered forms resemble staphylococci and coccobacilli (cocci and
rods) in tissue and in culture.
-
- The cancer microbe has been described as existing within
the cell (intracellular) and outside the cell (extracellular). The bacteria
have a particular affinity for connective tissue. Diller showed that the
microbe could also live within the nucleus of the cell (intranuclear),
where it could conceivably alter the genome, the genetic material of the
cell. The smallest forms of the microbe are "filterable" and
virus-sized, and the largest forms of the bacteria in vivo are compatible
with what pathologists call "Russell bodies."
-
- I believe Russell bodies represent large cell-wall-deficient
forms of bacteria. They are comparable to bacterial growth forms which
microbiologists call "large bodies." For more information about
Russell bodies and Russell's "parasite of cancer," refer to
my internet article "The Russell body: The forgotten clue to the bacterial
cause of cancer," - and my youtube.com video entitled "The cancer
microbe and the Russell body." One can also Google the "microbiology
of cancer." Cancer bacteria have many characteristics of "cell
wall deficient L-forms." Some of the published research of icons in
this field of microbiology, such as Lida H Mattman and Gerald Domingue,
is available on the net.
-
- Carefully observed bacterial isolates from cancer are
notoriously difficult to classify into a particular species, due to their
pleomorphism. Or they may simply be regarded as common staphylococci, streptococci,
or as coccobacilli (corynebacteria, propionibacteria) of no particular
significance. Such isolates are generally considered as non-important
"contaminants" or as "secondary invaders" of cancerous
tissue. It is only by repeatedly comparing what is grown in vitro from
diseased tissue to what is observed in vivo, as well as acid-fast staining
of both, that can determine if one is dealing with a possible "cancer
microbe."
-
- Most physicians still vehemently reject the idea of cancer-causing
bacteria (with the recent exception of stomach cancer). In 1970 when Livingston
named her "hidden killer" cancer germ Progenitor cryptocides,
she infuriated cancer experts and the American Cancer Society for her
audacity in naming a cancer germ that didn't exist, and for devising new
treatments for her cancer patients. For many years until her death in 1990,
Livingston was widely regarded in the medical community as a "quack."
She is largely forgotten, along with other scientists who passionately
wrote about bacteria in cancer.
-
- The proposed bacterial "Star Cell"
-
- After meeting Virginia and the three women, my research
segued into searches for similar pleomorphic bacteria in vivo in non-cancerous
diseases, such as lupus and sarcoidosis. Later, when I became more confident
about my microscopic studies, I wanted to see if these microbes could
be found in cancer, so I undertook bacterial studies in Hodgkin's and
non-Hodgkin's lymphoma, breast cancer, and in "classic" (pre-AIDS)
Kaposi's sarcoma.
-
- In my years of microscopic study, I repeatedly encountered
characteristic tightly-packed formations of bacteria which can be identified,
assuming one is patient and examines slides under the highest power of
the microscope (oil immersion). I am tentatively suggesting a name for
these clusters of round bacteria that are packed into or around a cell.
For more than a century, various investigators have termed these tiny forms
as coccoid forms, cocci, micrococci, granules, globoid forms, spheres,
spore forms, spore balls, cell wall deficient bacteria, L-forms, mycoplasma,
and other names. Unfortunately, the terminology in this field is a mess,
but all these terms basically denote a small round form.
-
- I propose the term "star cluster cell," or
"star cell" for short, because the appearance of these groupings
in vivo suggests a resemblance to what astronomers call "globoidal
clusters" in which the stars are closely allied together in the heavens,
and appear as "dots" in the cosmos (Fig 4).
-
-
-

-
- Fig 4. STAR CLUSTER. Copyrighted photo (#DP012)
of a tight globular star cluster called M15, filled with ancient stars,
about 12 billion years old. Courtesy of Jason Ware (www.galaxyphoto.com).
Compare the various "clusters" of bacteria in vivo in the various
photos with the cosmic design of a star cluster.
-
-
-
- These bacterial clusters can infect a cell in small numbers,
or they can be so tightly-packed into a cell that they obscure the nucleus.
In addition, the coccoid forms may appear as scattered extracellular forms,
far removed from any cell. I observed star cells in breast cancer (Fig
5), lung cancer (Fig 6) and prostate cancer (Fig 7). Scattered extracellular
variably-sized coccoid forms in a "milky way" pattern are shown
in the fatty layer of the skin in panniculitis (Fig 8).
-
-

-
- Fig 5. BREAST CANCER showing intracellular
coccoid forms in "star cell" formation. in Fite (acid-fast stain,
x1000, in oil)
-
-

-
- Fig 6. LUNG CANCER showing tightly packed
intracellular coccoid froms in the lung tumor. Fite (acid-fast) stain,
x1000
Click
Here For larger Image
-
-

-
- Fig 7. PROSTATE CANCER. A closely-knit focus
of blue-stained non-acid-fast coccoid forms in the cancerous prostate.
Fite (acid-fast) stain, x1000
- Click Here For larger Image
-
-

-
- Fig 8. PANNICULITIS. Inflammation of the fat
portion of the skin in a case of disabling pansclerotic morphea, a scleroderma-like
disease. Note the scattered "milky way" appearance of the individual
variably-sized non-acid-fast coccoid forms in the fatty layer. Fite (acid-fast)
stain, x1000
- Click Here For larger Image
-
-
- Individual coccoid forms can vary in size. In the late
1970s, my mentor Florence Seibert advised me to examine autopsy tissue
of patients dying of scleroderma. She reasoned that if bacteria are involved
in scleroderma skin, they should also be present in other organs in patients
dying from this disease; and this would strengthen my claims of a bacterial
etiology. Her suggestion led me (with the aid of several sympathetic pathologists)
to study autopsy tissue and to discover pleomorphic acid-fast bacteria
in patients dying from scleroderma, lupus erythematosus, Hodgkin's lymphoma,
Kaposi's sarcoma, mycosis fungoides (a form of T-cell lymphoma), and AIDS.
-
- Livingston sometimes referred to the cancer microbe
as a "connective tissue parasite." At death, these coccoid forms,
particularly in the connective tissue, can sometimes appear larger than
they do in skin biopsies, suggesting they "plump-up" as the
disease progresses to death. Plump star cells are seen in the connective
tissue autopsy slides from lupus (Fig 9) and from Hodgkin's lymphoma Fig
10). Autopsy tissue examinations are a fertile area for further investigations
into the role of bacteria in diseases of unknown etiology.
-
-
-

-
- Fig 9 . LUPUS ERYTHEMATOSUS. Two "star
cells" in the connective tissue seen in an autopsy specimen. Fite
(acid-fast) stain, x1000
- Click Here For larger Image
-
-
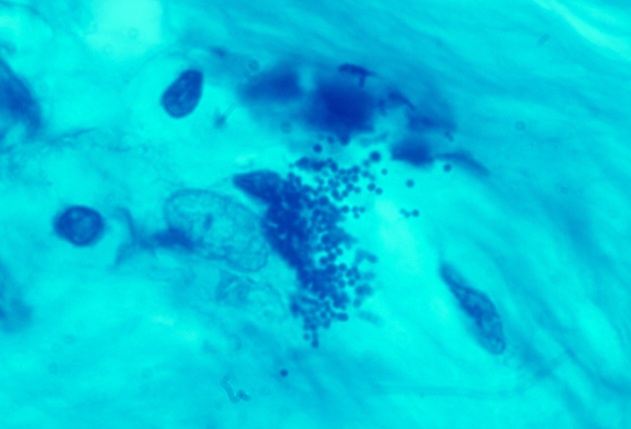
-
- Fig 10. HODGKIN'S LYMPHOMA showing plump
intra- and extracellular plump coccoid forms in the connective tissue at
autopsy. Fite (acid-fast) stain, x1000
- Click Here For larger Image
-
-
- The cancer microbe in AIDS
-
- Although HIV is almost universally accepted as the "sole
cause of AIDS," my microscopic examinations of AIDS-damaged tissue
indicates that bacteria play a crucial (and largely unrecognized) role
in the progression of HIV infection into "full-blown" AIDS. HIV
infection also leads to an increase in certain cancers in AIDS patients;
and typical and atypical mycobacterial infection is common in AIDS patients
worldwide, due to the extreme immunosuppression characteristic of AIDS.
-
- In 1981, the year the AIDS epidemic became official,
I reported variably acid-fast bacteria in "classic" (pre-AIDS)
Kaposi's sarcoma (KS) cases. An autopsied case of KS, conducted with the
assistance of a pathologist, also revealed similar bacteria post mortem
(Cantwell and Lawson, 1981). When AIDS in gay men first appeared I was
anxious to test their AIDS-damaged tissue and KS for acid-fast bacteria.
Fig 11 shows two typical star cells in an enlarged lymph node diagnosed
as "non-specific hyperplasia" in this reported AIDS case (Cantwell,
1982).
-
-

-
-
- Fig 11. LYMPH NODE (AIDS). Two foci of coccoid
forms ('star cells') in an enlarged AIDS-related lymph node reported
as "non-specific hyperplasia." Fite (acid-fast) stain, x1000
-
-
-
-
- In 1983, a year before the discovery of HIV, I reported
variably acid-fast bacteria in AIDS-related KS in two gay men; and also
myriads of similar bacteria in an autopsied case of AIDS and KS (Cantwell,
1983). Coccoid forms are numerous in skin tumors of AIDS-related KS (Fig
12).
-
-

-
-
- Fig 12. AIDS-RELATED KAPOSI'S SARCOMA showing
tightly-packed intracellular coccoid forms in the dermis of the skin tumor
in "star cluster' formation. Fite (acid-fast) stain, x1000
-
-
-
- In 1986 I detected rare acid-fast rods within a facial
tumor diagnosed as an "immunoblastic sarcoma" (a "B cell"
lymphoma tumor arising in the connective tissue) in an AIDS patient (Fig
13). A pleomorphic, atypical Mycobacterium avium-intracellulare (MAC)
was cultured from the tumor (Fig 14). In my experience, coccoid forms are
most likely to be easily encountered in vivo in scleroderma and in AIDS-damaged
tissue.
-
-

-
- Fig 13. AIDS-RELATED IMMUNOBLASTIC SARCOMA
showing three extremely rare acid-fast rod-shaped bacteria (in the center
of the photo) in vivo in the tumor. Mycobacterium avium-intracellulare
was cultured from the lesion in this fatal case. Fite (acid-fast) stain,
x1000.
-
-
-

-
- Fig 14. PLEOMORPHIC MYCOBACTERIUM AVIUM-INTRACELLULARE.
Smear of culture from AIDS-related immunoblastic lymphoma. Most forms are
red-purple acid-fast rods. However, notice the blue-staining round coccus
forms in the center of the photo. Ziehl-Neelsen (acid-fast) stain.
-
-
-
- The enigmatic coccal form of acid-fast mycobacteria
-
- I was taught in medical school that bacteria simply reproduce
by splitting in half, but controversial studies of mycobacteria
suggest these bacteria have a complex "life cycle." Such a view
is widely considered anathema. As a result, pathologists only recognize
the acid-fast rod of the TB germ - and ignore other reported pleomorphic
forms of mycobacteria.
-
- When country doctor Robert Koch first discovered the
rod forms of M. tuberculosis in 1882, he also noted "granules"
in the bacillus. These TB granules (also known coccoid forms) received
little attention until Hans Much began studying them in 1907. He discovered
these Gram-positive staining granules in suspected TB fluids in which Koch's
acid-fast rods could not be found. Remarkably, he learned these non-acid-fast
granules could "revert" into typical acid-fast rods. (For details
on the enigmatic "Much's granules," read the 1932 paper on the
net by Franklin R Miller regarding "induced development of non-acid-fast
forms of bacillus tuberculosis and other mycobacteria.")
-
- Fortunately, back in the 1970s, Eleanor Alexander-Jackson
kept prodding me to study "cell wall deficient" forms of
mycobacteria, which appear totally unlike the rod-shaped tubercle bacillus.
Little did I know that I was opening a Pandora's Box of strange microbes,
which would eventually lead to finding this type of germ in autoimmune
disease, cancer, and even AIDS.
-
- I was fascinated by an obscure 1964 report by Anna Csillag,
entitled "The mycococcus form of mycobacteria" (also posted
on the net). It helped me understand the curious relationship between
the ubiquitous coccoid forms in scleroderma and in culture, and their relation
to the acid-fast rod forms which were so exceedingly difficult to detect
in skin biopsies.
-
- Csillag's mycococcus was grown from M. tuberculosis and
was strikingly similar to ordinary micrococci (i.e. staphylococci), suggesting
that " a number of so-called micrococci belong in fact to the mycococci."
I bought a stock culture of "mycococcus" from the American Type
Culture Collection to compare with the coccoid forms in scleroderma (Fig
15). To my mind, they were indistinguishable.
-
-

-
- Fig 15. MYCOCOCCUS. The controversial coccus
form of mycobacteria derived from the acid-fast microbe that causes human
tuberculosis. Note the similarity to the coccoid forms observed in vivo
in the various diseases cited here. Ziehl-Neelsen (acid-fast) stain, x1000
- Click Here For larger Image
-
-
- In a 1987 report in Tubercle, entitled "Much's
granules revisited," medical microbiologist John L Stanford MD wrote:
"It is now 80 years since Hans Much published two of the most controversial
papers even written about tubercle bacilli. In them he described the appearances
of organisms that have come to be called Much's granules. Whether they
exist, whether they are a form of tubercle bacillus, and whether they can
replicate are questions that have never been settled completely. Out of
fashion for many years, the possibility of their existence is skated over
by most modern mycobacteriologists, yet it might be said that the more
we learn of the tubercle bacillus, the more we need Much's granules to
explain our findings. Our inability to find the causes of sarcoidosis [a
TB-like disease] and a number of other idiopathic diseases, and our inability
to grow the leprosy bacillus, would all gain a new dimension if Much's
granules really existed."
-
- In 1993, a DNA analysis by de Wit and Mitchison indicated
that mycococci derived from mycobacteria did not exist. Traag et al (2009)
also found no evidence that mycobacteria produced free-living "spores"
(i.e cocci). But challenging this research were reports by J Ghosh et al
in 2009, and by B Singh et al in 2010, showing that mycobacteria were indeed
able to produce "spores."
-
- Although this scientific controversy might appear esoteric,
an understanding of the complex life forms of mycobacteria is essential
when considering the proposed microbiology of cancer and diseases like
scleroderma, lupus, sarcoidosis, AIDS, and even tuberculosis itself. I
still don't understand why scientists and doctors can't agree (after a
century) whether the granule/coccus form of mycobacteria exists or not,
particularly when the TB germ infects billions of people. It is estimated
that one-third of the 7 billion people on the planet are infected with
this germ. How many people will get cancer? It is now estimated that 31%
of people worldwide will suffer some form of cancer.
-
- Cell wall deficient forms (L-forms) of mycobacteria
-
- A 1992 study of cell wall deficient bacteria by Chandresekhar
and Ratnam concluded that "acid-fast mycobacteria are converted into
non-acid-fast variants which remain dormant, only to revert to the parent
acid-fast bacilli in immunocompromised hosts, thence ultimately producing
disease."
-
- There are plenty of research studies showing that mycobacteria
exhibit extreme pleomorphism and can exist in vivo and in vitro as cell
wall deficient L-forms, lacking a fully-developed cell wall. According
to Lilia Michailova of the Institute of Microbiology, in Sofia, Bulgaria,
these forms vary in terms of their acid-fastness, and are undetectable
with ordinary stains (such as the routine hematoxylin-eosin stain used
by pathologists).
-
- In an internet interview with Amy Proal of the Autoimmunity
Research Foundation, Nadya Markova MD, a colleague of Michailova's at the
Institute, bemoans the difficulty of getting this type of research published
in journals because few people actually understand and want to accept these
investigations. She notes that physicians unfortunately don't pay enough
attention to the role of L-form bacteria in chronic diseases, and accept
them with difficulty.
-
- Some personal thoughts on cancer microbe research
-
- The rejection of cancer microbe research should be reconsidered
in light of new developments in 21st century microbiology. We now know
there are an estimated ten trillion cells in the human body; and 90%
of these cells are microbial cells, mainly bacterial cells. Some researchers
now refer to the human body as a "superorganism." Yet, no serious
consideration is given these largely unstudied body bacteria as possible
cancer-causing agents or agents of chronic disease.
-
- Recently, because of my old panniculitis research in
the 60s, I was asked to examine the skin biopsy of an unusual case. I
had previously suggested that an acid-fast stain be done, which was subsequently
reported by the pathologist as negative for acid-fast bacteria. After studying
the stained slide for fifty minutes under oil immersion (magnification,
x1000), I detected one cell filled with purple coccoid forms. After another
hour of study, I detected ten more bacterial cluster cells in the deep
dermis and in the fat. Lending credence to the role of acid-fast bacteria
in diseases of the fat, is a 2006 report by Neyrolles et al claiming that
TB germs hide from the immune system in the fat cells.
-
- I showed the intracellular coccoid cells to three dermatologists,
none of whom had seen this type of star-cell formation before. I doubt
I convinced any of them that these cells were indicative of bacteria, particularly
in light of the negative appraisal by the pathologist. I also couldn't
imagine many busy dermatologists (except for the most curious) spending
hours peering into a microscope to search for "star cells" of
dubious significance.
-
- This may explain, in part, why there has been no confirmation
(or denial) of my bacterial findings over the past five decades. Twenty
years ago, one pathologist admitted he was aware of the coccoid formations,
but did not want to report them because their precise significance
was not known.
-
- Proposing the name "star cell" will perhaps
offend some pathologists, but perhaps it will encourage people to search
for these bacterial clusters in acid-fast stained slides. As the photos
here indicate, the star cell is "real," although the interpretation
of its significance will undoubtedly cause controversy. These acid-fast
forms are not stain artifacts or "nuclear debris," or "secondary
invaders" or "opportunistic infections" because they are
so consistently found in conjunction with pathologic changes.
-
- Cell wall bacteria are notoriously resistant to antibiotics.
I am sometimes asked how to kill these infective forms I have reported
in tissue, but I have no clue how to put these bacteria back into harmony
with the body. I believe these bacterial clusters are a vital part of the
human body; and therefore impossible to eradicate totally. In that sense,
they are the indestructible and immortal primordial elements of life that,
like the star clusters in the heavens, cannot be destroyed.
-
- There is an ancient saying in Hermetic astrology that
states: "As above, so below." The axiom tries to explain why
man is the microcosm of the universe. Stressing the concept that the visible
stars in the sky are linked to life on earth, it was believed the microcosm
and macrocosm are intimately connected.
-
- What is the origin of the ubiquitous coccoid forms found
in vivo? There is some evidence connecting these forms to the origin of
life. (See my internet article: "Bacteria, cancer, and the origin
of life.") Fossilized bacteria are now indicating to scientists how
life may have evolved on planet Earth.
-
- Further study of these primordial forms in vivo in man
is desperately needed to determine how these microbes affect the life,
health, disease and death of the human "superorganism." Perhaps
the "star cell" will also reveal new connections between the
microcosm and the macrocosm, as the ancients believed. After all, "star
cells" are really "us."
-
- [Dr. Cantwell is a retired dermatologist and the author
of THE CANCER MICROBE and FOUR WOMEN AGAINST CANCER, both available from
Amazon.com]
-
- An extensive bibliography on the microbiology of cancer
is available on request. Email: alancantwell@sbcglobal.net. Website: www.ariesrisingpress.com
|














